Abstract
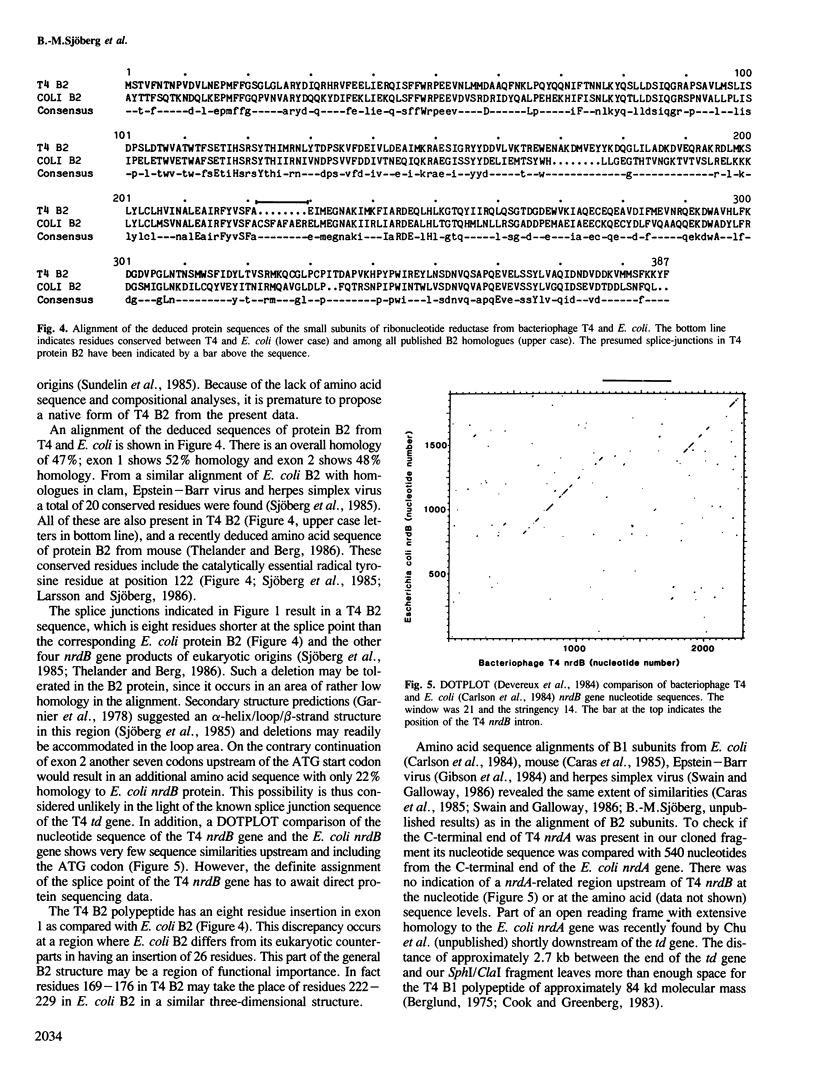
The bacteriophage T4 gene nrdB codes for the small subunit of the enzyme ribonucleotide reductase. The T4 nrdB gene was localized between 136.1 kb and 137.8 kb in the T4 genetic map according to the deduced structural homology of the protein to the amino acid sequence of its bacterial counterpart, the B2 subunit of Escherichia coli. This positions the C-terminal end of the T4 nrdB gene approximately 2 kb closer to the T4 gene 63 than earlier anticipated from genetic recombinational analyses. The most surprising feature of the T4 nrdB gene is the presence of an approximately 625 bp intron which divides the structural gene into two parts. This is the second example of a prokaryotic structural gene with an intron. The first prokaryotic intron was reported in the nearby td gene, coding for the bacteriophage T4-specific thymidylate synthase enzyme. The nucleotide sequence at the exon-intron junctions of the T4 nrdB gene is similar to that of the junctions of the T4 td gene: the anticipated exon-intron boundary at the donor site ends with a TAA stop codon and there is an ATG start codon at the putative downstream intron-exon boundary of the acceptor site. In the course of this work the denA gene of T4 (endonuclease II) was also located.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Belfort M., Pedersen-Lane J., Ehrenman K., Chu F. K., Maley G. F., Maley F., McPheeters D. S., Gold L. RNA splicing and in vivo expression of the intron-containing td gene of bacteriophage T4. Gene. 1986;41(1):93–102. doi: 10.1016/0378-1119(86)90271-4. [DOI] [PubMed] [Google Scholar]
- Belfort M., Pedersen-Lane J., West D., Ehrenman K., Maley G., Chu F., Maley F. Processing of the intron-containing thymidylate synthase (td) gene of phage T4 is at the RNA level. Cell. 1985 Jun;41(2):375–382. doi: 10.1016/s0092-8674(85)80010-6. [DOI] [PubMed] [Google Scholar]
- Belfort M., Pedersen-Lane J., West D., Ehrenman K., Maley G., Chu F., Maley F. Processing of the intron-containing thymidylate synthase (td) gene of phage T4 is at the RNA level. Cell. 1985 Jun;41(2):375–382. doi: 10.1016/s0092-8674(85)80010-6. [DOI] [PubMed] [Google Scholar]
- Berglund O., Karlström O., Reichard P. A new ribonucleotide reductase system after infection with phage T4. Proc Natl Acad Sci U S A. 1969 Mar;62(3):829–835. doi: 10.1073/pnas.62.3.829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berglund O. Ribonucleoside diphosphate reductase induced by bacteriophage T4. III. Isolation and characterization of proteins B1 and B2. J Biol Chem. 1975 Sep 25;250(18):7450–7455. [PubMed] [Google Scholar]
- Biggin M. D., Gibson T. J., Hong G. F. Buffer gradient gels and 35S label as an aid to rapid DNA sequence determination. Proc Natl Acad Sci U S A. 1983 Jul;80(13):3963–3965. doi: 10.1073/pnas.80.13.3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caras I. W., Levinson B. B., Fabry M., Williams S. R., Martin D. W., Jr Cloned mouse ribonucleotide reductase subunit M1 cDNA reveals amino acid sequence homology with Escherichia coli and herpesvirus ribonucleotide reductases. J Biol Chem. 1985 Jun 10;260(11):7015–7022. [PubMed] [Google Scholar]
- Carlson J., Fuchs J. A., Messing J. Primary structure of the Escherichia coli ribonucleoside diphosphate reductase operon. Proc Natl Acad Sci U S A. 1984 Jul;81(14):4294–4297. doi: 10.1073/pnas.81.14.4294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cech T. R. RNA splicing: three themes with variations. Cell. 1983 Oct;34(3):713–716. doi: 10.1016/0092-8674(83)90527-5. [DOI] [PubMed] [Google Scholar]
- Chu F. K., Maley G. F., Belfort M., Maley F. In vitro expression of the intron-containing gene for T4 phage thymidylate synthase. J Biol Chem. 1985 Sep 5;260(19):10680–10688. [PubMed] [Google Scholar]
- Chu F. K., Maley G. F., Maley F., Belfort M. Intervening sequence in the thymidylate synthase gene of bacteriophage T4. Proc Natl Acad Sci U S A. 1984 May;81(10):3049–3053. doi: 10.1073/pnas.81.10.3049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chua N. H., Blomberg F. Immunochemical studies of thylakoid membrane polypeptides from spinach and Chlamydomonas reinhardtii. A modified procedure for crossed immunoelectrophoresis of dodecyl sulfate.protein complexes. J Biol Chem. 1979 Jan 10;254(1):215–223. [PubMed] [Google Scholar]
- Clark R. W., Wever G. H., Wiberg J. S. High-molecular-weight DNA and the sedimentation coefficient: a new perspective based on DNA from T7 bacteriophage and two novel forms of T4 bacteriophage. J Virol. 1980 Jan;33(1):438–448. doi: 10.1128/jvi.33.1.438-448.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook K. S., Greenberg G. R. Properties of Bacteriophage T4 ribonucleoside diphosphate reductase subunits coded by nrdA and nrdB mutants. J Biol Chem. 1983 May 25;258(10):6064–6072. [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson S., Gräslund A., Skog S., Thelander L., Tribukait B. Cell cycle-dependent regulation of mammalian ribonucleotide reductase. The S phase-correlated increase in subunit M2 is regulated by de novo protein synthesis. J Biol Chem. 1984 Oct 10;259(19):11695–11700. [PubMed] [Google Scholar]
- Evans T., Rosenthal E. T., Youngblom J., Distel D., Hunt T. Cyclin: a protein specified by maternal mRNA in sea urchin eggs that is destroyed at each cleavage division. Cell. 1983 Jun;33(2):389–396. doi: 10.1016/0092-8674(83)90420-8. [DOI] [PubMed] [Google Scholar]
- Garnier J., Osguthorpe D. J., Robson B. Analysis of the accuracy and implications of simple methods for predicting the secondary structure of globular proteins. J Mol Biol. 1978 Mar 25;120(1):97–120. doi: 10.1016/0022-2836(78)90297-8. [DOI] [PubMed] [Google Scholar]
- Gibson T., Stockwell P., Ginsburg M., Barrell B. Homology between two EBV early genes and HSV ribonucleotide reductase and 38K genes. Nucleic Acids Res. 1984 Jun 25;12(12):5087–5099. doi: 10.1093/nar/12.12.5087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karn J., Matthes H. W., Gait M. J., Brenner S. A new selective phage cloning vector, lambda 2001, with sites for XbaI, BamHI, HindIII, EcoRI, SstI and XhoI. Gene. 1984 Dec;32(1-2):217–224. doi: 10.1016/0378-1119(84)90049-0. [DOI] [PubMed] [Google Scholar]
- Larsson A., Sjöberg B. M. Identification of the stable free radical tyrosine residue in ribonucleotide reductase. EMBO J. 1986 Aug;5(8):2037–2040. doi: 10.1002/j.1460-2075.1986.tb04461.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messing J., Vieira J. A new pair of M13 vectors for selecting either DNA strand of double-digest restriction fragments. Gene. 1982 Oct;19(3):269–276. doi: 10.1016/0378-1119(82)90016-6. [DOI] [PubMed] [Google Scholar]
- Mileham A. J., Revel H. R., Murray N. E. Molecular cloning of the T4 genome; organization and expression of the frd--DNA ligase region. Mol Gen Genet. 1980;179(2):227–239. doi: 10.1007/BF00425449. [DOI] [PubMed] [Google Scholar]
- Niggemann E., Green I., Meyer H. P., Rüger W. Physical mapping of bacteriophage T4. Mol Gen Genet. 1981;184(2):289–299. doi: 10.1007/BF00272920. [DOI] [PubMed] [Google Scholar]
- Olsson A., Moks T., Uhlén M., Gaal A. B. Uniformly spaced banding pattern in DNA sequencing gels by use of field-strength gradient. J Biochem Biophys Methods. 1984 Nov;10(1-2):83–90. doi: 10.1016/0165-022x(84)90053-8. [DOI] [PubMed] [Google Scholar]
- Rand K. N., Gait M. J. Sequence and cloning of bacteriophage T4 gene 63 encoding RNA ligase and tail fibre attachment activities. EMBO J. 1984 Feb;3(2):397–402. doi: 10.1002/j.1460-2075.1984.tb01819.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray P., Sinha N. K., Warner H. R., Snustad D. P. Genetic location of a mutant of bacteriophage T4 deficient in the ability to induce endonuclease II. J Virol. 1972 Jan;9(1):184–186. doi: 10.1128/jvi.9.1.184-186.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahlin M., Gräslund A., Ehrenberg A., Sjöberg B. M. Structure of the tyrosyl radical in bacteriophage T4-induced ribonucleotide reductase. J Biol Chem. 1982 Jan 10;257(1):366–369. [PubMed] [Google Scholar]
- Sjöberg B. M., Eklund H., Fuchs J. A., Carlson J., Standart N. M., Ruderman J. V., Bray S. J., Hunt T. Identification of the stable free radical tyrosine residue in ribonucleotide reductase. A sequence comparison. FEBS Lett. 1985 Apr 8;183(1):99–102. doi: 10.1016/0014-5793(85)80962-5. [DOI] [PubMed] [Google Scholar]
- Staden R. A computer program to enter DNA gel reading data into a computer. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 2):499–503. doi: 10.1093/nar/12.1part2.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staden R. Automation of the computer handling of gel reading data produced by the shotgun method of DNA sequencing. Nucleic Acids Res. 1982 Aug 11;10(15):4731–4751. doi: 10.1093/nar/10.15.4731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Standart N. M., Bray S. J., George E. L., Hunt T., Ruderman J. V. The small subunit of ribonucleotide reductase is encoded by one of the most abundant translationally regulated maternal RNAs in clam and sea urchin eggs. J Cell Biol. 1985 Jun;100(6):1968–1976. doi: 10.1083/jcb.100.6.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundelin J., Melhus H., Das S., Eriksson U., Lind P., Trägårdh L., Peterson P. A., Rask L. The primary structure of rabbit and rat prealbumin and a comparison with the tertiary structure of human prealbumin. J Biol Chem. 1985 May 25;260(10):6481–6487. [PubMed] [Google Scholar]
- Swain M. A., Galloway D. A. Herpes simplex virus specifies two subunits of ribonucleotide reductase encoded by 3'-coterminal transcripts. J Virol. 1986 Mar;57(3):802–808. doi: 10.1128/jvi.57.3.802-808.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trimble R. B., Galivan J., Maley F. The temporal expression of T2r + bacteriophage genes in vivo and in vitro. Proc Natl Acad Sci U S A. 1972 Jul;69(7):1659–1663. doi: 10.1073/pnas.69.7.1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waring R. B., Davies R. W. Assessment of a model for intron RNA secondary structure relevant to RNA self-splicing--a review. Gene. 1984 Jun;28(3):277–291. doi: 10.1016/0378-1119(84)90145-8. [DOI] [PubMed] [Google Scholar]
- Warner H. R., Snustad P., Jorgensen S. E., Koerner J. F. Isolation of bacteriophage T4 mutants defective in the ability to degrade host deoxyribonucleic acid. J Virol. 1970 Jun;5(6):700–708. doi: 10.1128/jvi.5.6.700-708.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson G. G., Tanyashin V. I., Murray N. E. Molecular cloning of fragments of bacteriophage T4 DNA. Mol Gen Genet. 1977 Nov 14;156(2):203–214. doi: 10.1007/BF00283493. [DOI] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- Yeh Y. C., Dubovi E. J., Tessman I. Control of pyrimidine biosynthesis by phage T4: mutants unable to catalyze the reduction of cytidine diphosphate. Virology. 1969 Apr;37(4):615–623. doi: 10.1016/0042-6822(69)90279-7. [DOI] [PubMed] [Google Scholar]
- Yeh Y. C., Tessman I. Control of pyrimidine biosynthesis by phage T4. II. In vitro complementation between ribonucleotide reductase mutants. Virology. 1972 Mar;47(3):767–772. doi: 10.1016/0042-6822(72)90567-3. [DOI] [PubMed] [Google Scholar]


