Abstract
The incidence of heart transplants in the USA has increased by 85.8% since 2011, resulting in a growing population of recipients requiring long-term immunosuppressive therapy. While essential for preventing organ rejection, this therapy significantly increases melanoma risk. This meta-analysis investigates the incidence and risk factors of melanoma in heart transplant recipients. A systematic review and meta-analysis were conducted following Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines, including observational studies reporting melanoma incidence in heart transplant recipients. Relative risk (RR) was synthesized from standardized incidence ratios, hazard ratios, incidence rate ratios, and standardized mortality ratios. The meta-analysis incorporated 10 studies, including 22 415 heart transplant recipients. The pooled RR was 2.21 (95% confidence interval: 1.32–3.71; P = 0.003), indicating a significantly elevated melanoma risk. This study highlights the critical need for preventive dermatological strategies in heart transplant recipients and calls for further research into the impact of different immunosuppressive regimens on melanoma risk. Despite limitations, these findings offer valuable insights for optimizing long-term patient care.
Keywords: epidemiology, heart transplant, immunosuppressive therapy, melanoma, meta-analysis
Introduction
The number of heart transplants in the USA has risen dramatically, increasing by 85.8% since 2011 and reaching 3668 adult transplants in 2022 [1]. While these life-saving procedures are becoming more common, they bring with them significant challenges, particularly the long-term use of immunosuppressive therapy. This therapy is crucial for preventing graft rejection but also weakens the immune system, making transplant recipients more susceptible to various cancers, including melanoma.
Melanoma, the most lethal type of skin cancer, is the fifth most common malignancy in both men and women in the USA, with an estimated 97 610 new cases and 7990 deaths expected in 2023 [2]. The risk of melanoma is particularly concerning for transplant recipients due to their immunosuppressed state. While kidney transplant recipients are known to have a significantly elevated risk of developing melanoma – up to eight times higher than the general population [3] – similar risks are suspected in heart transplant recipients, who also endure high-dose and prolonged immunosuppressive regimens.
Given the rising number of heart transplants and the critical need to manage posttransplant cancer risks, understanding the incidence and risk factors for melanoma in heart transplant patients is essential. This study aims to fill this gap, offering insights that could lead to improved monitoring and management strategies for this vulnerable population.
Materials and methods
Data source and searches
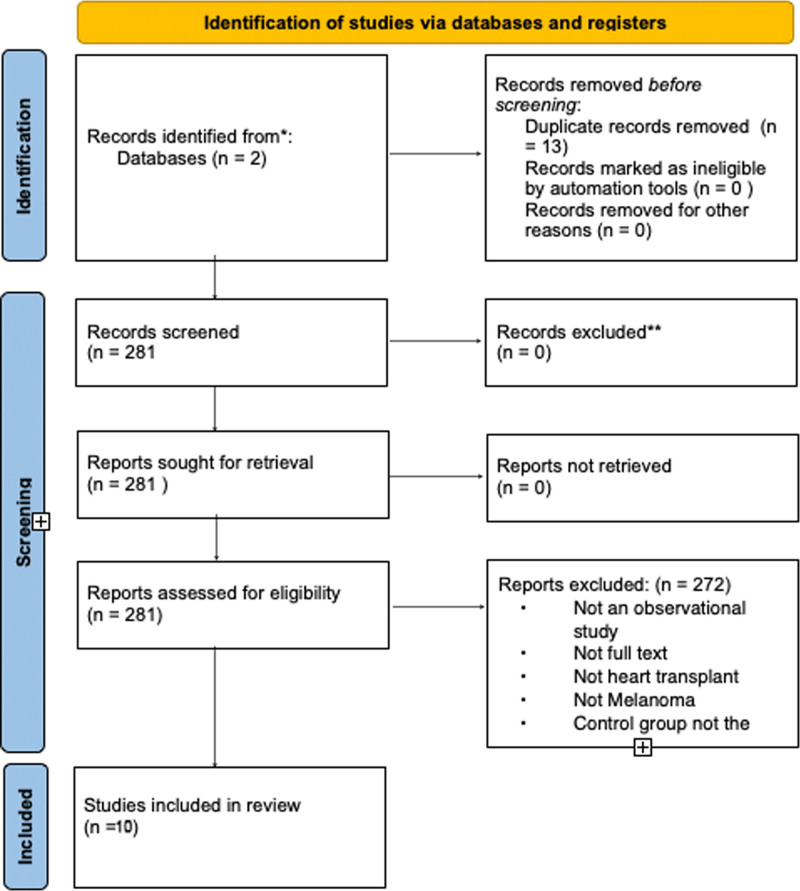
We followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines and have summarized our search process in Fig. 1. Adhering to the PRISMA checklist, we ensured comprehensive and transparent reporting of our systematic review [4] (Supplementary Data 1, Supplemental digital content 1, http://links.lww.com/MR/A412). Two investigators (P.C. and A.K.) developed the search strategy, which was revised and approved by the other investigators. We searched the following databases until 31 July 2024: PubMed-MEDLINE and EMBASE-OVID. The PubMed search strategy is shown in Supplementary Data 2, Supplemental digital content 2, http://links.lww.com/MR/A413 File. There was no language limitation.
Fig. 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA).
Study selection
We included only observational studies reporting the incidence of melanoma after patients received a heart transplant. We excluded studies that did not match our target population, did not report incidence of melanoma specifically in patients with a heart transplant, or did not compare the incidence to the general population. Three investigators (P.C., A.K., and M.S.) independently screened each record title and abstract for potential inclusion. They then assessed full texts of selected abstracts. Discrepancies were resolved through discussion or by another investigator (M.G.).
Outcomes
In this meta-analysis, we exclusively focused on assessing the relative risk (RR) of melanoma in heart transplant patients compared to the general population. Our primary outcome measure was the standardized incidence ratio (SIR), hazard ratio, incidence rate ratio (IRR), and standardized mortality ratio (SMR), all of which were converted to a common metric of RR for uniformity in analysis. No other outcomes, such as absolute risk, morbidity, mortality, or other health-related metrics, were evaluated in this study.
Data extraction
Three investigators (P.C., A.K., and M.S.) independently extracted the following variables from the studies: location, study type (e.g. retrospective cohort), study period, immunosuppressant regimen, comparison population, percentage of male participants, age range, median follow-up duration, number of heart transplant patients, number of male/female melanoma cases, number of melanoma cases, measure of effect (CI), log(RR), and SE(logRR). Discrepancies in data extraction were resolved through discussion or by consulting a fourth investigator (M.G.).
Risk of bias assessment
Two investigators (P.C. and A.K.), independently assessed risk of bias (RoB) by using the ROBINS-I (Risk Of Bias In Non-Randomized Studies of Interventions) tool for cohorts [5]. Disagreements were resolved by discussion with a third investigator (M.S.). ROBINS-I evaluates seven bias domains: due to confounding, selection of participants into the study, classification of interventions, deviations from intended interventions, missing outcome data, measurement of the outcome, and selection of the reported result. Risk of bias assessment can be viewed in Supplementary Data 3, Supplemental digital content 3, http://links.lww.com/MR/A414.
Statistical analysis
We reported our systematic review according to the 2020 PRISMA guidelines (Supplementary Data 1, Supplemental digital content 1, http://links.lww.com/MR/A412) [4]. The meta package of RevMan was used for all meta-analyses [6]. Inverse variance random effect meta-analyses were performed to evaluate the RR of melanoma in heart transplant patients compared to the general population. Effects of the meta-analyses were reported as log-transformed relative risk (logRR) for consistency across different types of risk measures (e.g. SIR, hazard ratio, IRR, SMR). These log-transformed measures were then exponentiated to present the results as RR. Heterogeneity of effects among studies was quantified with the I2 statistic, with an I2 > 60% indicating high heterogeneity. A leave-one-out sensitivity analysis was conducted to assess the robustness of the overall results. In this analysis, each study was sequentially excluded, and the meta-analysis was repeated to determine the influence of each individual study on the pooled effect size and heterogeneity. This process helped identify any studies that had a disproportionate impact on the overall findings. The quality of evidence will be evaluated using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) methodology, which covers five aspects: RoB, inconsistency, indirectness, imprecision, and publication bias [7]. Publication bias was assessed using Egger’s test [8].
Results
Selection of studies
A comprehensive search was conducted on 31 July 2024, using PubMed and EMBASE to identify relevant citations. The search included Medical Subject Headings terms ‘Heart Transplant’ and ‘Melanoma’ or ‘Skin Neoplasms’ to capture studies involving melanoma incidence in heart transplant patients compared to the general population. The population, intervention, comparison, outcome (PICO) system on EMBASE was similarly used with terms ‘Heart Graft’ and ‘Melanoma’ or ‘Skin Cancer’ along with study types like randomized controlled trial (RCT), observational, retrospective, and prospective.
A total of 294 citations were identified, with 192 from PubMed and 102 from EMBASE. Following this, 13 duplicates were removed, leaving 281 results for additional screening. During the screening process, studies were excluded based on criteria such as not being an RCT or an observational study, not providing full text, not focusing on heart transplant, not addressing melanoma, or not having a control group of the general population. Ultimately, 10 full-text studies were selected for inclusion. The included studies’ design can be seen in Table 1.
Table 1.
Study design and characteristics
| Study | Location | Study type | Study period | Immunosuppressant regimen | Comparison population | Male (%) | Age range | Follow-up (median years) |
|---|---|---|---|---|---|---|---|---|
| Krynitz et al. [11] | Sweden | Retrospective cohort | 1970–2008 | – | Swedish population (SIR) | – | 9 to 75 | 5.1 |
| Jensen et al. [20] | Denmark | Retrospective cohort | 1977–2006 | Azathioprine, cyclosporine, and prednisolone | Danish population (SIR) | 368 (80%) | Median age at transplant 50 y/o | 5 |
| Jiang et al. [12] | Canada | Retrospective cohort | 1981–1998 | – | Canadian population (SIR) | 1575 (82.5%) | <10 to >60 | 4.8 |
| Kellerman et al. [15] | New York | Retrospective cohort | 1994–2007 | Calcineurin inhibitor, prednisone, MMF | US population (SIR) | 658 (77%) | 50 to 66 | 5.3a |
| Na et al. [13] | Australia | Retrospective cohort | 1984–2006 | – | Australia population (SIR) | 1218 (80.2%) | 0 to >60 | 5 |
| Robbins et al. [16] | USA | Retrospective cohort | 1987–2010 | Azathioprine | US population (SIR) | – | 0 to >65 | 4 |
| Secnikova et al. [14] | Czech Republic | Retrospective cohort | 1993–2010 | Cyclosporine A or tacrolimus, MMF or azathioprine | Czech population (SMR) | 493 (81.7%) | 34.9 to 61.3 | 6.4 |
| Fattouh et al. [23] | France | Retrospective cohort | 1991–2015 | Long-term corticosteroids, cyclosporine, mycophenolate mofetil | French population (SIR) | – | 9 to 75 | 4.4 |
| Jäämaa-Holmberg et al. [19] | Finland | Retrospective cohort | 1985–2014 | Cyclosporine (before 2008) or tacrolimus (2008 onward), azathioprine (before 2002) or mycophenolate (2002 onward) | General Finnish population (SIR) | 381 (79.5%) | Median age at transplant 52 y/o | 7.8 |
| Liu et al. [22] | Taiwan | Retrospective cohort | 2000–2015 | Calcineurin inhibitor (cyclosporine/tacrolimus), antimetabolite (azathioprine/mycophenolate mofetil), mTOR inhibitor (sirolimus/everolimus), combinations of these drugs | National Health Insurance Research Database (NHIRD) in Taiwan | – | 35.5 to 60.5 | 10.12 ± 9.7 |
MMF, mycophenolate mofetil; mTOR, mechanistic target of rapamycin; SIR, standardized incidence ratio; SMR, standardized mortality ratio.
Represents a mean (average).
Characteristics of included studies
The general characteristics of the included retrospective cohort studies are detailed in Table 1. All included studies were published across various periods and countries, providing a comprehensive view of melanoma risk in heart transplant patients. The studies spanned different regions, including Sweden, Denmark, Canada, New York, Australia, the USA, the Czech Republic, France, Finland, and Taiwan. The study periods ranged from as early as 1970 to as recent as 2015.
Immunosuppressant regimens varied widely among the studies, including combinations of azathioprine, cyclosporine, prednisolone, calcineurin inhibitors, prednisone, mycophenolate mofetil (MMF), tacrolimus, and long-term corticosteroids. It is important to note that the studies did not specifically report the number of heart transplant patients on these immunosuppressant drugs. Instead, we extracted this data manually from papers that discussed immunosuppressant usage in overall organ transplant populations.
The comparison populations were predominantly national or regional populations, and the method of comparison included SIR, SMR, IRR, and hazard ratios. The total number of heart transplant patients included across all studies was 22 415. However, not every study provided specific male/female ratios for the heart transplant population; we gathered the available data from the studies that did report this information, which was too low to do a formal analysis on.
The median follow-up duration across the studies ranged from 4 to 10.12 years, allowing for substantial observation periods to assess melanoma incidence. The male percentage varied from 77% to 82.5% in studies that reported gender distribution. Age ranges of participants varied broadly, with some studies reporting specific age ranges, such as 50–66 years, and others providing median ages at transplant.
A total of 579 melanoma cases were identified across the studies. Table 2 summarizes the number of heart transplant patients, male/female melanoma cases, and the number of melanoma cases.
Table 2.
Study outcome
| Study | Heart transplant patients (N = 22 415) | Male/female melanoma cases | Cases of melanoma (N = 579) | Measure of effect (CI) | Log(RR) | SE(LogRR) |
|---|---|---|---|---|---|---|
| Krynitz et al. [11] | 557 | – | 4 | SIR 2.4 (0.6–6.1) | 0.875 | 0.592 |
| Jensen et al. [20] | 459 | – | 1 | SIR 1.8 (CI 0.1–10) | 0.588 | 1.175 |
| Jiang et al. [12] | 1703 | – | 5 | SIR 2.8 (CI 0.9–6.5) | 1.03 | 0.504 |
| Kellerman et al. [15] | 851 | 5/0 | 5 | SIR 3.1 (CI 0.99–7.1) | 1.131 | 0.503 |
| Na et al. [13] | 1518 | – | 29 | SIR 3.04 (CI 2.03–4.36) | 1.112 | 0.195 |
| Robbins et al. [16] | 16, 325 | – | 519 | IRR 0.91 (CI 0.69–1.21) | −0.094 | 0.143 |
| Secnikova et al. [14] | 603 | 2/1 | 3 | SMR 2.5 (CI 0.7–6.73) | 0.916 | 0.577 |
| Fattouh et al. [23] | – | – | 6 | SIR 0.87 (CI 0.17–1.57) | −0.139 | 0.567 |
| Jäämaa-Holmberg et al. [19] | 479 | – | 5 | SIR 3.8 (1.2–8.8) | 1.335 | 0.508 |
| Liu et al. [22] | 878 | – | 2 | 4.983 h (1.195–25.047) | 1.606 | 0.776 |
CI, confidence interval; IRR, incidence rate ratio; RR, relative risk; SIR, standardized incidence ratio; SMR, standardized mortality ratio.
Risk of bias of included studies
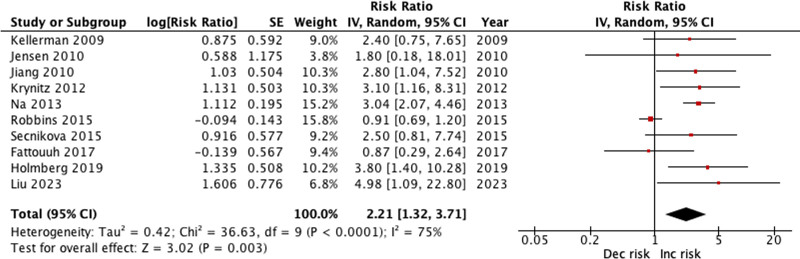
The leave-one-out sensitivity analysis revealed that the overall heterogeneity, as measured by the I2 statistic, remained high across most exclusions, indicating substantial variability among the studies (Supplementary Data 3, Supplemental digital content 3, http://links.lww.com/MR/A414). Notably, the exclusion of Robbins 2015 significantly reduced the I2 to 0%, suggesting that this study had a substantial impact on the overall heterogeneity. Additionally, excluding Robbins et al., 2015 resulted in an increase in the overall effect size from 2.21 [1.32–3.71] to 2.80 [2.12–3.70], highlighting the significant influence this study had on the pooled effect estimate. This indicates that the study by Robbins et al. contributed not only to the heterogeneity but also affected the magnitude of the estimated risk.
Risk of bias was evaluated using the The ROBINS-I assessment for non-randomized controlled trials (Supplementary Data 3, Supplemental digital content 3, http://links.lww.com/MR/A414). The studies generally exhibit low risk across most domains, particularly in the selection of participants, classification of interventions, measurement of outcomes, deviations from intended interventions, and selection of reported results. These domains benefit from the use of comprehensive national registries and standardized data, which ensure accurate and complete data collection. However, the risk of confounding is noted as serious in some studies [3,4] due to potential differences in immunosuppressive regimens and underlying health conditions. The overall RoB is moderate for most studies, reflecting these confounding concerns while acknowledging the robustness of data handling in other areas.
The GRADE analysis for the RR of melanoma in heart transplant patients, based on 10 observational studies, indicates a low certainty of evidence. Initially rated as low due to the observational nature of the studies, the evidence quality was further downgraded due to high heterogeneity (I2 = 75%). There were no downgrades for RoB, as assessed by ROBINS-I, or for imprecision, indirectness, and publication bias, the latter confirmed by Egger’s test showing no significant publication bias (z = 0.908, P = 0.364). Despite the large magnitude of effect [RR = 2.21, 95% confidence interval (CI): 1.32–3.71], there was no evidence to support an upgrade for a dose–response gradient or to account for confounding. Thus, the overall certainty of the evidence remains low (Supplementary Data 3, Supplemental digital content 3, http://links.lww.com/MR/A414).
Outcomes
When compared to the general population, heart transplant patients showed a statistically significant increased risk of melanoma, with a RR of 2.21 (95% CI: 1.32–3.71, P = 0.003, I2 = 75%), as depicted in Fig. 2.
Fig. 2.
Risk of melanoma forest plot.
Discussion
This meta-analysis confirms that heart transplant recipients are at a significantly elevated risk of developing melanoma compared to the general population. The pooled RR of 2.21 found in this study aligns with previous research documenting increased cancer susceptibility in organ transplant recipients due to immunosuppressive therapy.
The heightened risk of melanoma in heart transplant recipients is largely attributable to the prolonged and high-dose use of immunosuppressants, such as calcineurin inhibitors, which impair DNA repair mechanisms and promote mutagenesis. Specifically, calcineurin inhibitors have been shown to inhibit nucleotide excision repair, a critical pathway for repairing ultraviolet (UV)-induced DNA damage, thereby increasing the likelihood of mutagenesis and oncogenesis in skin cells. Studies have also demonstrated that these drugs can activate oncogenic pathways, such as the phosphatidylinositol (3,4,5)-trisphosphate/protein kinase B or murine thymoma viral oncoprotein homolog and mitogen-activated protein kinase pathways, which are known to drive melanoma development by promoting cell proliferation and survival while suppressing the body’s natural tumor surveillance mechanisms [8]. Moreover, the type and duration of immunosuppressive therapy appear to play a significant role in melanoma risk. Calcineurin inhibitors, in particular, have been implicated in increased melanoma risk compared to other immunosuppressants such as mechanistic target of rapamycin inhibitors, which may have a more favorable safety profile concerning cancer risk [9,10]. However, we were unable to analyze these differences because some studies did not specify which patients in our study population were on particular medications [11–13].
It is important to consider the potential impact of gender differences among heart transplant recipients on melanoma risk. Since men are more prone to heart conditions that may ultimately lead to heart transplantation, studies often show a higher proportion of male participants. This gender imbalance in heart transplant rates may contribute to the observed higher incidence of melanoma in men compared to women in studies that report gender-specific data [14,15]. To truly understand whether there is a genuine difference in melanoma risk between sexes, further research should focus on controlling for these disparities, examining factors such as hormonal influences, differences in immunosuppressive therapies, and variations in sun protection behaviors.
The variability in melanoma incidence across different geographic locations highlights the influence of environmental factors, particularly UV exposure, on melanoma risk. Studies have shown that individuals living in regions with higher UV exposure, such as Australia and the southern USA, are at greater risk of developing melanoma. This aligns with findings from Ref. [16], which suggest potential geographical variations in cancer incidence posttransplantation. This increased risk underscores the necessity for stringent preventive measures, including the use of broad-spectrum sunscreens and protective clothing, as well as regular dermatological assessments for transplant recipients in these regions [17,18]. The geographic variability also emphasizes the importance of considering local environmental factors when developing melanoma screening and prevention strategies for heart transplant recipients since increased incidence in melanoma posttransplant is not limited to regions with increased UV exposure [19,20].
The long latency period for melanoma development posttransplantation, which can extend up to a decade, further emphasizes the need for sustained dermatological surveillance in heart transplant recipients. Regular skin checks and early detection are critical for improving outcomes, as early stage melanoma is more amenable to treatment. Current clinical guidelines, such as those from the American Academy of Dermatology and other relevant transplant organizations, recommend annual dermatological assessments for transplant recipients [21]. Patient education on photoprotection, including the use of sunscreens and avoidance of peak UV times, and lifestyle modifications could further reduce melanoma risk.
Limitations
Despite the valuable insights gained from this meta-analysis, several limitations should be acknowledged. First, the retrospective nature of the included studies introduces inherent limitations related to data accuracy and completeness. Second, there was substantial variability in the immunosuppressant regimens reported across the studies. The wide range of drugs and combinations used, including azathioprine, cyclosporine, prednisolone, calcineurin inhibitors, prednisone, MMF, tacrolimus, and long-term corticosteroids, complicates the ability to draw definitive conclusions about the impact of specific regimens on melanoma risk. Moreover, the studies did not consistently report the exact number of heart transplant patients on each immunosuppressant regimen. This introduces potential inaccuracies and limits the ability to perform detailed subgroup analyses.
Third, the comparison populations used in the studies varied, with some studies employing national or regional populations and others using different comparison metrics such as SIR, SMR, IRR, and hazard ratios [22]. For the study with Fattouh et al., to calculate the SIR for melanoma in heart transplant patients, I derived the expected number of cases using the known SIR for renal transplant patients and the observed melanoma cases in that group. I then calculated the SIR for heart transplant patients by comparing the observed cases in this population to the derived expected cases [23]. The variability in comparison methods likely contributed to the high heterogeneity observed in the meta-analysis, as indicated by the I2 statistic. The leave-one-out sensitivity analysis revealed that excluding the Robbins et al., 2015 study significantly reduced heterogeneity (I2 = 0%) and increased the overall effect size from a RR of 2.21–2.80. This suggests that the Robbins study had a substantial impact on both the variability and the magnitude of the estimated risk, underscoring the need for caution when interpreting the pooled estimates.
Finally, the GRADE analysis indicated a low certainty of evidence, primarily due to the observational nature of the included studies and the high level of heterogeneity. These limitations highlight the need for further prospective studies with standardized reporting of immunosuppressive regimens and detailed demographic data to better understand the melanoma risk in heart transplant patients and to refine the strategies for monitoring and managing this risk.
Conclusion
In conclusion, this meta-analysis reinforces the significant melanoma risk faced by heart transplant recipients and underscores the importance of regular monitoring and tailored preventive strategies. By addressing the identified risk factors and implementing personalized care approaches, it may be possible to reduce the incidence and improve the management of melanoma in this high-risk population.
Acknowledgements
Conflicts of interest
There are no conflicts of interest.
Supplementary Material
Footnotes
Supplemental Digital Content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's website, www.melanomaresearch.com.
References
- 1.Colvin MM, Smith JM, Ahn YS, Handarova DK, Martinez AC, Lindblad KA, et al. OPTN/SRTR 2022 annual data report: heart. Am J Transplant 2024; 24:S305–S393. [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clin 2023; 73:17–48. [DOI] [PubMed] [Google Scholar]
- 3.Bouwes Bavinck N, Hardie DR, Green A, Cutmore S, MacNaught A, O’Sullivan B, et al. The risk of skin cancer in renal transplant recipients in Queensland, Australia: a follow-up study. Transplantation 1996; 61:715–721. [DOI] [PubMed] [Google Scholar]
- 4.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Moher D. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021; 372:n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sterne JAC, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, Higgins JPT. ROBINS-I: a tool for assessing risk of bias in non-randomized studies of interventions. BMJ 2016; 355:i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.The Cochrane Collaboration. Review Manager (RevMan) [Computer software]. Version 5.4. The Cochrane Collaboration; 2020. https://training.cochrane.org/online-learning/core-software-cochrane-reviews/revman. [Google Scholar]
- 7.Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008; 336:924–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guo W, Wang H, Li C. Signal pathways of melanoma and targeted therapy. Signal Transduct Target Ther 2021; 6:424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kauffman HM, Cherikh WS, Cheng Y, Hanto DW, Kahan BD. Maintenance immunosuppression with target-of-rapamycin inhibitors is associated with a reduced incidence of de novo malignancies. Transplantation 2005; 80:883–889. [DOI] [PubMed] [Google Scholar]
- 10.Kulbat A, Richter K, Stefura T, Kołodziej-Rzepa M, Kisielewski M, Wojewoda T, Wysocki WM. Systematic review of calcineurin inhibitors and incidence of skin malignancies after kidney transplantation in adult patients: a study of 309,551 cases. Curr Oncol 2023; 30:5727–5737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Krynitz B, Edgren G, Lindelöf B, Baecklund E, Brattström C, Wilczek H, Smedby KE. Risk of skin cancer and other malignancies in kidney, liver, heart and lung transplant recipients 1970 to 2008—a Swedish population-based study. Int J Cancer 2013; 132:1429–1438. [DOI] [PubMed] [Google Scholar]
- 12.Jiang Y, Villeneuve PJ, Wielgosz A, Schaubel DE, Fenton SS, Mao Y. The incidence of cancer in a population-based cohort of Canadian heart transplant recipients. Am J Transplant 2010; 10:637–645. [DOI] [PubMed] [Google Scholar]
- 13.Na R, Grulich AE, Meagher NS, McCaughan GW, Keogh AM, Vajdic CM. Comparison of de novo cancer incidence in Australian liver, heart and lung transplant recipients. Am J Transplant 2013; 13:174–183. [DOI] [PubMed] [Google Scholar]
- 14.Secnikova Z, Gopfertova D, Hoskova L, Hercogova J, Dzambova M, Jirakova A, et al. Significantly higher incidence of skin cancer than other malignancies in patients after heart transplantation. A retrospective cohort study in the Czech Republic. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub 2015; 159:648–651. [DOI] [PubMed] [Google Scholar]
- 15.Kellerman L, Neugut A, Burke B, Mancini D. Comparison of the incidence of de novo solid malignancies after heart transplantation to that in the general population. Am J Cardiol 2009; 103:562–566. [DOI] [PubMed] [Google Scholar]
- 16.Robbins HA, Clarke CA, Arron ST, Tatalovich Z, Kahn AR, Hernandez BY, et al. Melanoma risk and survival among organ transplant recipients. J Invest Dermatol 2015; 135:2657–2665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mittal A, Colegio OR. Skin cancers in organ transplant recipients. Am J Transplant 2017; 17:2509–2530. [DOI] [PubMed] [Google Scholar]
- 18.Ascha M, Ascha MS, Tanenbaum J, Bordeaux JS. Risk factors for melanoma in renal transplant recipients. JAMA Dermatol 2017; 153:1130–1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jäämaa-Holmberg S, Salmela B, Lemström K, Pukkala E, Lommi J. Cancer incidence and mortality after heart transplantation – a population-based national cohort study. Acta Oncol 2019; 58:859–863. [DOI] [PubMed] [Google Scholar]
- 20.Jensen AO, Svaerke C, Farkas D, Pedersen L, Kragballe K, Sørensen HT. Skin cancer risk among solid organ recipients: a nationwide cohort study in Denmark. Acta Derm Venereol 2010; 90:474–479. [DOI] [PubMed] [Google Scholar]
- 21.Crow LD, Jambusaria-Pahlajani A, Chung CL, Baran DA, Lowenstein SE, Abdelmalek M, et al. Initial skin cancer screening for solid organ transplant recipients in the United States: Delphi method development of expert consensus guidelines. Transpl Int 2019; 32:1268–1276. [DOI] [PubMed] [Google Scholar]
- 22.Liu S, Wang W, Chiang C, Chung C, Tsao C, Chien W, Hung C. Risk of skin cancer in kidney, liver and heart recipients: a nationwide population-based study in Taiwan. Indian J Dermatol Venereol Leprol 2022; 89:372–377. [DOI] [PubMed] [Google Scholar]
- 23.Fattouh K, Ducroux E, Decullier E, Kanitakis J, Morelon E, Boissonnat P, et al. Increasing incidence of melanoma after solid organ transplantation: a retrospective epidemiological study. Transpl Int 2017; 30:1172–1180. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.