Abstract
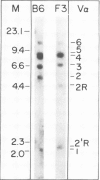
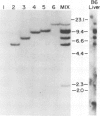
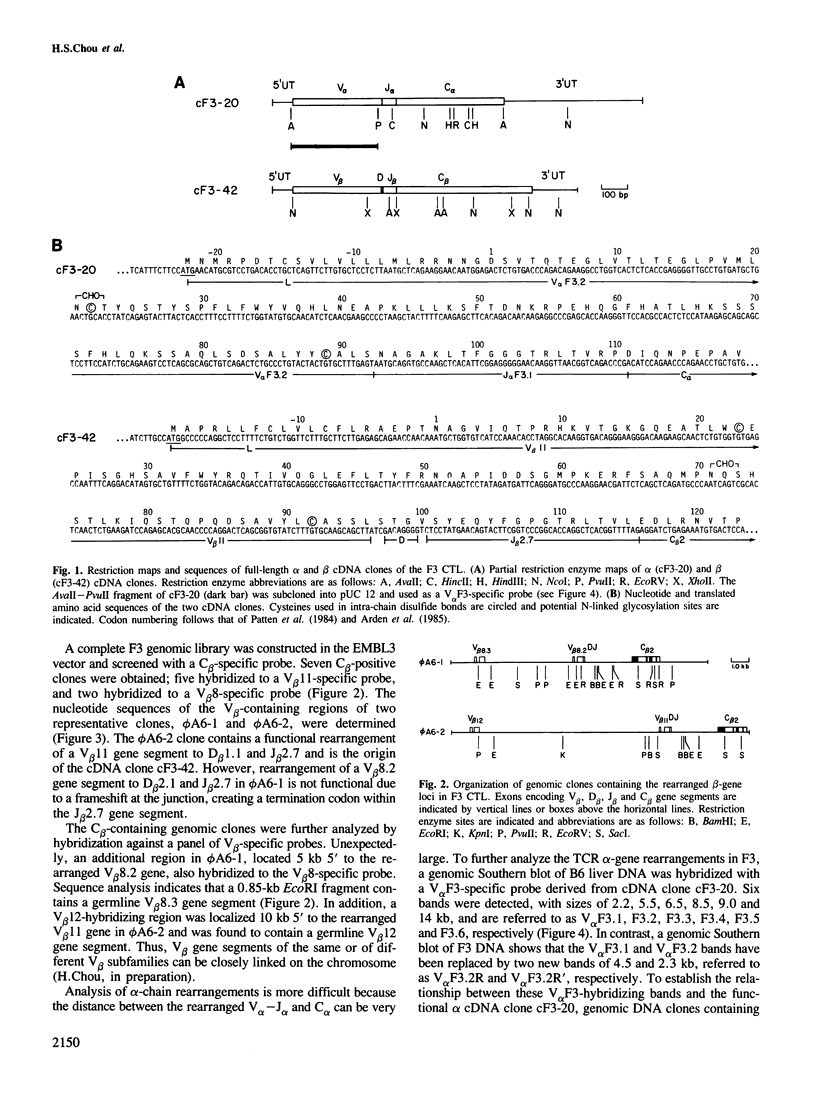
Both cDNA and genomic clones of the T cell receptor (TCR) alpha- and beta-chain genes of the alloreactive cytotoxic T lymphocyte (CTL) clone F3 were examined. Two distinct rearrangement events, one functional and one non-functional, were found for both the alpha and beta loci. Thus only a single functional TCR alpha beta heterodimer could be defined, consistent with allelic exclusion in the TCR genes. The V alpha gene employed by F3 is part of a six-member V alpha subfamily. Genomic clones containing each member of this subfamily were isolated and the V alpha nucleotide sequences determined. Five of these six genes are functional; these genes differ from each other by 7-14% at the amino acid level. A single dominant hypervariable region was defined within this subfamily, in contrast to the pattern of variability seen between V alpha genes in general.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alt F. W., Baltimore D. Joining of immunoglobulin heavy chain gene segments: implications from a chromosome with evidence of three D-JH fusions. Proc Natl Acad Sci U S A. 1982 Jul;79(13):4118–4122. doi: 10.1073/pnas.79.13.4118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arden B., Klotz J. L., Siu G., Hood L. E. Diversity and structure of genes of the alpha family of mouse T-cell antigen receptor. 1985 Aug 29-Sep 4Nature. 316(6031):783–787. doi: 10.1038/316783a0. [DOI] [PubMed] [Google Scholar]
- Barth R. K., Kim B. S., Lan N. C., Hunkapiller T., Sobieck N., Winoto A., Gershenfeld H., Okada C., Hansburg D., Weissman I. L. The murine T-cell receptor uses a limited repertoire of expressed V beta gene segments. Nature. 1985 Aug 8;316(6028):517–523. doi: 10.1038/316517a0. [DOI] [PubMed] [Google Scholar]
- Becker D. M., Pattern P., Chien Y., Yokota T., Eshhar Z., Giedlin M., Gascoigne N. R., Goodnow C., Wolf R., Arai K. Variability and repertoire size of T-cell receptor V alpha gene segments. Nature. 1985 Oct 3;317(6036):430–434. doi: 10.1038/317430a0. [DOI] [PubMed] [Google Scholar]
- Behlke M. A., Chou H. S., Huppi K., Loh D. Y. Murine T-cell receptor mutants with deletions of beta-chain variable region genes. Proc Natl Acad Sci U S A. 1986 Feb;83(3):767–771. doi: 10.1073/pnas.83.3.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentley D. L., Rabbitts T. H. Evolution of immunoglobulin V genes: evidence indicating that recently duplicated human V kappa sequences have diverged by gene conversion. Cell. 1983 Jan;32(1):181–189. doi: 10.1016/0092-8674(83)90508-1. [DOI] [PubMed] [Google Scholar]
- Benton W. D., Davis R. W. Screening lambdagt recombinant clones by hybridization to single plaques in situ. Science. 1977 Apr 8;196(4286):180–182. doi: 10.1126/science.322279. [DOI] [PubMed] [Google Scholar]
- Bevan M. J. Alloimmune cytotoxic T cells: Evidence that they recognize serologically defined antigens and bear clonally restricted receptors. J Immunol. 1975 Jan;114(1 Pt 2):316–319. [PubMed] [Google Scholar]
- Bothwell A. L., Paskind M., Reth M., Imanishi-Kari T., Rajewsky K., Baltimore D. Heavy chain variable region contribution to the NPb family of antibodies: somatic mutation evident in a gamma 2a variable region. Cell. 1981 Jun;24(3):625–637. doi: 10.1016/0092-8674(81)90089-1. [DOI] [PubMed] [Google Scholar]
- Chien Y. H., Gascoigne N. R., Kavaler J., Lee N. E., Davis M. M. Somatic recombination in a murine T-cell receptor gene. Nature. 1984 May 24;309(5966):322–326. doi: 10.1038/309322a0. [DOI] [PubMed] [Google Scholar]
- Chien Y., Becker D. M., Lindsten T., Okamura M., Cohen D. I., Davis M. M. A third type of murine T-cell receptor gene. Nature. 1984 Nov 1;312(5989):31–35. doi: 10.1038/312031a0. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Coleclough C., Perry R. P., Karjalainen K., Weigert M. Aberrant rearrangements contribute significantly to the allelic exclusion of immunoglobulin gene expression. Nature. 1981 Apr 2;290(5805):372–378. doi: 10.1038/290372a0. [DOI] [PubMed] [Google Scholar]
- Crews S., Griffin J., Huang H., Calame K., Hood L. A single VH gene segment encodes the immune response to phosphorylcholine: somatic mutation is correlated with the class of the antibody. Cell. 1981 Jul;25(1):59–66. doi: 10.1016/0092-8674(81)90231-2. [DOI] [PubMed] [Google Scholar]
- Dembić Z., Haas W., Weiss S., McCubrey J., Kiefer H., von Boehmer H., Steinmetz M. Transfer of specificity by murine alpha and beta T-cell receptor genes. Nature. 1986 Mar 20;320(6059):232–238. doi: 10.1038/320232a0. [DOI] [PubMed] [Google Scholar]
- Desiderio S. V., Yancopoulos G. D., Paskind M., Thomas E., Boss M. A., Landau N., Alt F. W., Baltimore D. Insertion of N regions into heavy-chain genes is correlated with expression of terminal deoxytransferase in B cells. Nature. 1984 Oct 25;311(5988):752–755. doi: 10.1038/311752a0. [DOI] [PubMed] [Google Scholar]
- Early P., Hood L. Allelic exclusion and nonproductive immunoglobulin gene rearrangements. Cell. 1981 Apr;24(1):1–3. doi: 10.1016/0092-8674(81)90492-x. [DOI] [PubMed] [Google Scholar]
- Frischauf A. M., Lehrach H., Poustka A., Murray N. Lambda replacement vectors carrying polylinker sequences. J Mol Biol. 1983 Nov 15;170(4):827–842. doi: 10.1016/s0022-2836(83)80190-9. [DOI] [PubMed] [Google Scholar]
- Goverman J., Minard K., Shastri N., Hunkapiller T., Hansburg D., Sercarz E., Hood L. Rearranged beta T cell receptor genes in a helper T cell clone specific for lysozyme: no correlation between V beta and MHC restriction. Cell. 1985 Apr;40(4):859–867. doi: 10.1016/0092-8674(85)90345-9. [DOI] [PubMed] [Google Scholar]
- Gubler U., Hoffman B. J. A simple and very efficient method for generating cDNA libraries. Gene. 1983 Nov;25(2-3):263–269. doi: 10.1016/0378-1119(83)90230-5. [DOI] [PubMed] [Google Scholar]
- Hayday A. C., Diamond D. J., Tanigawa G., Heilig J. S., Folsom V., Saito H., Tonegawa S. Unusual organization and diversity of T-cell receptor alpha-chain genes. 1985 Aug 29-Sep 4Nature. 316(6031):828–832. doi: 10.1038/316828a0. [DOI] [PubMed] [Google Scholar]
- Heilig J. S., Glimcher L. H., Kranz D. M., Clayton L. K., Greenstein J. L., Saito H., Maxam A. M., Burakoff S. J., Eisen H. N., Tonegawa S. Expression of the T-cell-specific gamma gene is unnecessary in T cells recognizing class II MHC determinants. Nature. 1985 Sep 5;317(6032):68–70. doi: 10.1038/317068a0. [DOI] [PubMed] [Google Scholar]
- Hood L., Kronenberg M., Hunkapiller T. T cell antigen receptors and the immunoglobulin supergene family. Cell. 1985 Feb;40(2):225–229. doi: 10.1016/0092-8674(85)90133-3. [DOI] [PubMed] [Google Scholar]
- Howe R. C., Russell J. H. Isolation of alloreactive CTL clones with cyclical changes in lytic activity. J Immunol. 1983 Nov;131(5):2141–2146. [PubMed] [Google Scholar]
- Ikuta K., Ogura T., Shimizu A., Honjo T. Low frequency of somatic mutation in beta-chain variable region genes of human T-cell receptors. Proc Natl Acad Sci U S A. 1985 Nov;82(22):7701–7705. doi: 10.1073/pnas.82.22.7701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kappler J., Kubo R., Haskins K., Hannum C., Marrack P., Pigeon M., McIntyre B., Allison J., Trowbridge I. The major histocompatibility complex-restricted antigen receptor on T cells in mouse and man: identification of constant and variable peptides. Cell. 1983 Nov;35(1):295–302. doi: 10.1016/0092-8674(83)90232-5. [DOI] [PubMed] [Google Scholar]
- Kronenberg M., Goverman J., Haars R., Malissen M., Kraig E., Phillips L., Delovitch T., Suciu-Foca N., Hood L. Rearrangement and transcription of the beta-chain genes of the T-cell antigen receptor in different types of murine lymphocytes. Nature. 1985 Feb 21;313(6004):647–653. doi: 10.1038/313647a0. [DOI] [PubMed] [Google Scholar]
- Kronenberg M., Siu G., Hood L. E., Shastri N. The molecular genetics of the T-cell antigen receptor and T-cell antigen recognition. Annu Rev Immunol. 1986;4:529–591. doi: 10.1146/annurev.iy.04.040186.002525. [DOI] [PubMed] [Google Scholar]
- Kunkel T. A., Gopinathan K. P., Dube D. K., Snow E. T., Loeb L. A. Rearrangements of DNA mediated by terminal transferase. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1867–1871. doi: 10.1073/pnas.83.6.1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loh D. Y., Bothwell A. L., White-Scharf M. E., Imanishi-Kari T., Baltimore D. Molecular basis of a mouse strain-specific anti-hapten response. Cell. 1983 May;33(1):85–93. doi: 10.1016/0092-8674(83)90337-9. [DOI] [PubMed] [Google Scholar]
- Malissen M., McCoy C., Blanc D., Trucy J., Devaux C., Schmitt-Verhulst A. M., Fitch F., Hood L., Malissen B. Direct evidence for chromosomal inversion during T-cell receptor beta-gene rearrangements. Nature. 1986 Jan 2;319(6048):28–33. doi: 10.1038/319028a0. [DOI] [PubMed] [Google Scholar]
- Maniatis T., Hardison R. C., Lacy E., Lauer J., O'Connell C., Quon D., Sim G. K., Efstratiadis A. The isolation of structural genes from libraries of eucaryotic DNA. Cell. 1978 Oct;15(2):687–701. doi: 10.1016/0092-8674(78)90036-3. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- McIntyre B. W., Allison J. P. The mouse T cell receptor: structural heterogeneity of molecules of normal T cells defined by xenoantiserum. Cell. 1983 Oct;34(3):739–746. doi: 10.1016/0092-8674(83)90530-5. [DOI] [PubMed] [Google Scholar]
- Ollo R., Rougeon F. Gene conversion and polymorphism: generation of mouse immunoglobulin gamma 2a chain alleles by differential gene conversion by gamma 2b chain gene. Cell. 1983 Feb;32(2):515–523. doi: 10.1016/0092-8674(83)90471-3. [DOI] [PubMed] [Google Scholar]
- Patten P., Yokota T., Rothbard J., Chien Y., Arai K., Davis M. M. Structure, expression and divergence of T-cell receptor beta-chain variable regions. Nature. 1984 Nov 1;312(5989):40–46. doi: 10.1038/312040a0. [DOI] [PubMed] [Google Scholar]
- Perlmutter R. M., Berson B., Griffin J. A., Hood L. Diversity in the germline antibody repertoire. Molecular evolution of the T15 VN gene family. J Exp Med. 1985 Dec 1;162(6):1998–2016. doi: 10.1084/jem.162.6.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raulet D. H., Garman R. D., Saito H., Tonegawa S. Developmental regulation of T-cell receptor gene expression. Nature. 1985 Mar 7;314(6006):103–107. doi: 10.1038/314103a0. [DOI] [PubMed] [Google Scholar]
- Roehm N. W., Carbone A., Kushnir E., Taylor B. A., Riblet R. J., Marrack P., Kappler J. W. The major histocompatibility complex-restricted antigen receptor on T cells: the genetics of expression of an allotype. J Immunol. 1985 Sep;135(3):2176–2182. [PubMed] [Google Scholar]
- Saito H., Kranz D. M., Takagaki Y., Hayday A. C., Eisen H. N., Tonegawa S. A third rearranged and expressed gene in a clone of cytotoxic T lymphocytes. Nature. 1984 Nov 1;312(5989):36–40. doi: 10.1038/312036a0. [DOI] [PubMed] [Google Scholar]
- Schnell H., Steinmetz M., Zachau H. G., Schechter I. An unusual translocation of immunoglobulin gene segments in variants of the mouse myeloma MPC11. Nature. 1980 Jul 10;286(5769):170–173. doi: 10.1038/286170a0. [DOI] [PubMed] [Google Scholar]
- Selsing E., Storb U. Somatic mutation of immunoglobulin light-chain variable-region genes. Cell. 1981 Jul;25(1):47–58. doi: 10.1016/0092-8674(81)90230-0. [DOI] [PubMed] [Google Scholar]
- Siu G., Kronenberg M., Strauss E., Haars R., Mak T. W., Hood L. The structure, rearrangement and expression of D beta gene segments of the murine T-cell antigen receptor. 1984 Sep 27-Oct 3Nature. 311(5984):344–350. doi: 10.1038/311344a0. [DOI] [PubMed] [Google Scholar]
- Snodgrass H. R., Dembić Z., Steinmetz M., von Boehmer H. Expression of T-cell antigen receptor genes during fetal development in the thymus. Nature. 1985 May 16;315(6016):232–233. doi: 10.1038/315232a0. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Tonegawa S. Somatic generation of antibody diversity. Nature. 1983 Apr 14;302(5909):575–581. doi: 10.1038/302575a0. [DOI] [PubMed] [Google Scholar]
- Wu T. T., Kabat E. A. An analysis of the sequences of the variable regions of Bence Jones proteins and myeloma light chains and their implications for antibody complementarity. J Exp Med. 1970 Aug 1;132(2):211–250. doi: 10.1084/jem.132.2.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Heijne G. Patterns of amino acids near signal-sequence cleavage sites. Eur J Biochem. 1983 Jun 1;133(1):17–21. doi: 10.1111/j.1432-1033.1983.tb07424.x. [DOI] [PubMed] [Google Scholar]