Abstract
Assisted reproductive technology (ART) pregnancies present a higher risk of singleton preterm birth than natural pregnancies, but the underlying molecular mechanism remains largely unknown. RNA m6A modification is a key epigenetic mechanism regulating cellular function, but the role of m6A modification, especially its “reader” YTHDC1, in preterm delivery remains undefined. To delineate the role and epigenetic mechanism of m6A modification in ART preterm delivery, the effects of YTHDC1 on trophoblastic function were evaluated by CCK-8, EdU, Transwell, and flow cytometry analyses post its overexpression or knockdown. Downstream signaling pathways of YTHDC1 were investigated by RNA-seq, and targeted mRNAs were explored by RIP-seq and MeRIP-seq. Upstream transcriptional factors of YTHDC1 were determined by ChIP-seq and luciferase reporter assays. Elevated YTHDC1 was detected in human ART-conceived preterm placentas and in murine preterm placentas post estradiol (E2) exposure. In vitro experiments showed that YTHDC1 promoted trophoblastic cell proliferation and migration, but inhibited cell apoptosis. Mechanistically, E2 was proven to upregulate YTHDC1 expression via retinoid X receptor alpha (RXRA) in trophoblastic cells. Enhanced YTHDC1 expression augmented the translation of RPL37 in an m6A-dependent manner by binding to m6A-modified RPL37 mRNA and concomitantly promoted the overall translational output. Importantly, administration of siRNA targeting YTHDC1 effectively delayed the progression of preterm delivery. In conclusion, the identified E2/RXRA/YTHDC1/RPL37 axis provides new insights into the epigenetic mechanism underlying ART-associated preterm delivery. The findings offer a potential prognostic biomarker and therapeutic target for preterm delivery.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00018-024-05467-x.
Keywords: Preterm delivery, Assisted reproductive technology, m6A, YTHDC1, Trophoblastic dysfunction
Introduction
Infertility affects approximately 10% of women of child-bearing age and has become an increasingly public health issue. Consequently, the use of assisted reproductive technology (ART) has steadily increased, with up to 4% of all births resulting from ART [1]. Compared to natural pregnancy, ART-conceived pregnancies face higher risks of adverse perinatal and long-term outcomes, such as preterm birth (PTB), low birth weight, and metabolic-related diseases [2]. Notably, PTB is the leading cause of death among children under 5 years old and can result in lifelong detrimental effects [3, 4]. Specifically, the rate of PTB was 14.9% for singleton infants conceived by ART, compared to 8.3% for all singleton infants [5]. However, there is currently no specific intervention for preterm delivery, underscoring the need for a comprehensive understanding of its etiopathogenesis, especially in the ART population.
Previous works have suggested that placental dysfunction is a major cause of premature birth [6]. The placenta primarily comprises trophoblasts, and abnormal migration and invasion of trophoblasts would lead to irregular spiral artery remodeling and impaired material exchange between the fetus and mother [7–10]. Trophoblastic cells undergo a well-coordinated sequential differentiation during pregnancy, accompanied by the apoptosis of certain trophoblastic cells. Abnormal apoptosis disrupts the balance of maternal-fetal interface immunity and hormone release, potentially triggering premature birth [11]. Therefore, homeostasis of trophoblastic function is vital for successful pregnancy. However, the ART process involves many unnatural exposures, such as high estrogen levels, controlled ovarian hyperstimulation, and embryo cryopreservation, which might disrupt gene regulation in trophoblastic cells [12]. Especially, high estrogen exposure can persist through early pregnancy [13]. Growing evidence suggests that adverse exposures during ART might cause lasting changes in epigenetic modifications [6, 12]. However, most studies on PTB focus on naturally conceived populations, leaving the mechanisms, particularly epigenetic mechanisms, of ART-conceived preterm delivery largely unexplored.
Proper epigenetic regulation is critical to trophoblastic function [14]. Epigenetic modifications primarily encompass DNA and RNA methylation, chromatin remodeling, and non-coding RNA regulation [14]. N6-Methyladenosine (m6A) RNA modification refers to adenosine methylation at the N6 position and represents the most frequent modification of eukaryotic mRNA. m6A modification is controlled by three classes of molecules: “writers” (METTL14, METTL3, WTAP, etc.), “erasers” (ALKBH5 and FTO), and “readers” (YTHDC1/2, HNRNPC, HNRNPA2B1, YTHDF1/2/3, IGF2BP1/2/3, eIF3, etc.) [15, 16]. m6A modification influences nearly all facets of RNA metabolism, such as splicing, decay, export, and translation [17–20]. YT521-B homology-domain-containing protein 1 (YTHDC1), a primary reader protein, plays a crucial role in numerous biological processes and is implicated in various diseases. For instance, in bladder cancer, decreased YTHDC1 expression inhibited proliferation and metastasis [21]. However, YTHDC1 expression varies among tissues and cell types. Research in preeclampsia and miscarriage has shown that m6A methylation can modulate trophoblastic cell function [22, 23]. Nevertheless, the precise role and associated mechanism of the m6A reader YTHDC1 in trophoblastic cells remain undefined.
In this study, we found that YTHDC1 was aberrantly up-regulated in ART preterm-birth placentas and was essential for trophoblastic cell proliferation, migration, and invasion. Multi-omics analysis identified the ribosomal large subunit protein RPL37 as the direct target of YTHDC1 in trophoblastic cells. YTHDC1 bound to m6A-modified RPL37 and fine-tuned RPL37 translation to further facilitate the overall protein translation in trophoblastic cells. Notably, administration of siRNA specifically targeting YTHDC1 effectively delayed the progression of preterm delivery.
Materials and methods
Clinical sample collection
The collection of human placentas was authorized by the Medical Ethics Committee of the International Peace Maternity and Child Health Hospital (IPMCH), affiliated with Shanghai Jiao Tong University School of Medicine (approval number: GKLW-2016-21). Written consent was secured from each participant before labor. The inclusion criteria were made for pregnant women who underwent ART at IPMCH between 2018 and 2021, aged between 20 and 40 years old. Exclusions were made for participants with complications such as preeclampsia, gestational diabetes, gestational hypertension, and chorioamnionitis. Placentas were processed within 1 h of delivery. Samples measuring 2 cm × 2 cm were excised from behind the site of cord insertion on the fetal surface. To reduce blood contamination, the samples were gently washed twice with sterile saline before being stored at -80 °C. The study utilized a total of 29 placentas, with 17 from ART-term pregnancies and 12 from ART-preterm pregnancies. The demographic characteristics of both ART-term and ART-preterm pregnancies, including maternal age, body mass index (BMI), gestational age at delivery, and mode of delivery, can be found in Supplementary Table S1.
Animal experiments
All animal experiments received approval from the Shanghai Jiao Tong University School of Medicine Institutional Animal Care & Use Committee (approval number: B-2022-015) and adhered to the approved guidelines. Specific pathogen-free C57BL/6J mice were allowed to acclimate to the facility for a minimum of two weeks before the start of the experiments and were mated at 8 weeks of age. Pregnancy was confirmed by the detection of a vaginal plug following overnight mating, which was designated as embryonic (E) 0.5 day (d).
For the high estradiol (E2) exposure model, mice were orally given 100 µg/kg/day of 17-β-estradiol (Y0001823; Sigma, Michigan, USA) dissolved in 100 µl of corn oil (Sigma) from E5.5d to E11.5d. Control mice received 100 µl/day of corn oil orally. Subsequently, mice were randomly divided into two groups: the preterm group and the full-term group. At E15.5d, the preterm group received a subcutaneous injection of 250 µg of RU-486 (Sigma) to induce preterm delivery, while the control group received a subcutaneous injection of 100 µl PBS. Placentas from the preterm group were collected at 12 h post-injection. Placentas from the full-term group were gathered at E18.5d.
For the in vivo siRNA model, either siYTHDC1 or siNC (RiboBio, Guangzhou, China) was administered intravenously through the tail vein at E13.5d and E15.5d. On E15.5d, pregnant mice were given a subcutaneous injection of 250 µg of RU-486 to initiate preterm birth. The onset of preterm labor was determined by the time elapsed from the administration of RU486 to the birth of the first pup. During the onset of labor, the mice were euthanized, and placentas were collected for subsequent analyses. Samples were either fixed in 4% paraformaldehyde (PFA) overnight or directly stored at -80 °C.
Isolation of primary human trophoblastic (PHT)cells
Placental villous tissues (about 30 g) were isolated from human term placentas following a well-established method [24]. The minced villous tissue was washed three times using PBS at room temperature, and subjected to sequential trypsin (Sigma Aldrich, MO, USA) and DNase I (Sigma Aldrich) digestions at 0.25% [24]. The suspensions were carefully layered over a Percoll density gradient and centrifuged. The middle layer, which contained a highly enriched population of viable mononuclear trophoblastic cells, was collected and washed with DMEM. The cells were then diluted to 0.5 × 106/ml with DMEM supplemented with 2 mM glutamine, 10% heat-inactivated fetal calf serum, 25 mM HEPES, 100 IU/ml penicillin, and 100 µg/ml streptomycin. The cells were plated in 6-well plates and incubated under low oxygen (5%) at 37 °C. By 3 days in culture, most cytotrophoblast cells have differentiated into syncytiotrophoblast [25]. Immunofluorescence analysis of obtained cells was performed with cytokeratin 7 (Santa Cruz Biotechnology) and DAPI (Sigma). Cells were cultured for 5 days for following experiments.
Cell culture
HTR-8/SVneo, a simian virus 40 large T antigen immortalized human normal trophoblastic cell line, was gifted by Dr. Charles H. Graham. Human choriocarcinoma cell line JAR and human embryonic kidney cell line HEK-293T cells were obtained from the National Collection of Authenticated Cell Cultures (Shanghai, China). HTR-8/SVneo cells were cultured in DMEM/F12 (Gibco, NY, USA), JAR cells were cultured in Roswell Park Memorial Institute medium (RPMI-1640, Gibco), and HEK-293T cells were cultured in DMEM (Gibco). Each medium was routinely supplemented with 10% fetal bovine serum (FBS, Hyclone, IL, USA). Cells were cultured at 37 °C in a 5% CO2 humidified atmosphere.
For E2 treatment, HTR-8/SVneo and JAR cells were treated with E2 (HY-B0141; MCE, Shanghai, China) at the concentrations of 0 (control), 1, 7.5, 10 nM for 24 h. The concentrations of E2 were selected according to previous reports [26, 27]. Cells were then harvested for Western blotting analysis.
siRNA transfection
All siRNAs or nonsense scramble siRNA (siNC) were synthesized by RiboBio. The sequences of all siRNAs are presented in Supplementary Table S2. HTR-8/SVneo or JAR cells were seeded into 6-well plates. Next day, cells with 50–60% confluency were transfected with 5 µl of siRNA or siNC (50 µM) using Lipofectamine RNAiMAX (Invitrogen, CA, USA) according to the manufacturer’s instructions. Cells were then harvested for further experiments at 48 h post transfection.
Plasmid construction and lentiviral infection
The sgRNA for YTHDC1 knockout was designed using an online tool (https://zlab.bio/guide-design-resources) and then cloned into the lentiCRISPR v2 vector (Addgene, Watertown, USA) for CRISPR editing. The sgRNA sequences were listed in Supplementary Table S3. The open reading frames of YTHDC1, RPL37, FTO, and RXRA were cloned into the lentivirus expression vector pCDH-CMV-MCS-EF1-Puro (pCDH) (Addgene). Plasmids were co-transfected into HEK-293T cells with the packaging vectors psPAX2 (Addgene) and pMD2.G (Addgene) by using Lipofectamine 2000 (Invitrogen). Following transfection, lentivirus particles were harvested after 48 h, filtered through 0.45 μm filters, and then transduced into cells. The primers were listed in Supplementary Table S3. All constructs were verified by DNA sequencing (Sangon Biotech, Shanghai, China).
Cell counting kit-8 (CCK-8) assay
Cells were seeded in 96-well plates in 100 µl of medium at a density of 5000 cells per well. CCK-8 reagent (MedChemExpress, Shanghai, China) was added into each well at 24, 72, and 120 h. After incubation for 1.5 h at 37 °C, the absorbance at 450 nm was measured using a microplate reader (BioTek, CA, USA).
Colony formation assay
At 48 h post transfection, 5000 cells per well were seeded in 6-well plates. Medium was refreshed on the third day post-seeding. Colonies were fixed with methanol for 30 min after 10 days culture for JAR cells and 12 days culture for HTR-8/SVneo cells, and then stained with 1% crystal violet (Sigma) for 15 min. Clone numbers were counted under an inverted microscope.
5-ethynyl-2’-deoxyuridine (EdU) assay
EdU assay was conducted using the Cell-Light EdU Apollo 567 In Vitro Kit (RiboBio) according to the manufacturer’s instructions. Briefly, the EdU reagent was added to JAR and HTR-8/SVneo cells seeded in 96-well plates at 48 h post transfection and cultured for 2 h at 37 °C. After twice washing with PBS, cells were fixed with 4% PFA for 30 min, incubated with 0.1% Triton-X 100 for 15 min, and then stained with Apollo 567 for 30 min at room temperature in the dark. Images were taken under an inverted microscope.
Apoptosis assay
After 48 h of transfection, JAR and HTR-8/SVneo cells were washed with cold PBS, harvested by trypsin digestion, and then were centrifuged at 4 °C, 1000 g for 5 min. Cells were stained using the Annexin V-FITC/PI Cell Apoptosis Kit (Yeasen, Shanghai, China) according to the manufacturer’s instructions. Apoptosis was analyzed on a flow cytometer (BD Bioscience, CA, USA).
Migration and invasion assays
The migratory and invasive capabilities of JAR and HTR-8/SVneo cells were investigated using 24-well Transwell plates (8 μm pore size; Corning, NY, USA). For the migration assay, 5 × 104 transfected cells suspended in 0.2 ml serum-free media were seeded in the Transwell upper chamber, and 0.6 ml RPMI-1640 or DMEM/F12 medium containing 10% FBS was added into the lower chamber. Cells were then cultured for 24 h. For the invasion assay, upper chamber was pre-coated with 50 µl Matrigel (BD Biosciences), and 5 × 104 transfected cells were seeded into the upper chamber in 0.2 ml serum-free medium and incubated for 36 h. Cells that had not migrated or invaded in the upper surface of the chambers were wiped off using a cotton swab. Cells were then fixed with methanol for 15 min and stained with 0.1% crystal violet for 30 min, and counted under a light microscope. Five randomly selected fields were scored per well. The mean cell number of the five fields was calculated to be the number of migrated or invaded cells.
Quantitative real-time PCR (qRT-PCR) and RNA-seq
Total RNA was extracted from tissues and cells using Trizol (Invitrogen) according to the manufacturer’s instructions. For qRT-PCR, RNA was reverse transcribed to cDNA by using a Reverse Transcription Kit (Accurate Biology, Changsha, China). Messenger RNA levels were quantified with a Quant Studio (TM) 7 Flex System (Applied Biosystems) using SYBR® Green PCR Master Mix (Accurate Biology). All samples were normalized to β-actin, and analyzed using the 2−ΔΔCt method. The primers (Supplementary Table S3) were designed by Primer-BLAST (NCBI). VAHTS mRNA-seq V3 Library Prep Kit (Vazyme, Nanjing, China) was utilized to construct the RNA-seq library. Sequencing was performed on the Illumina HiSeq 2500 platform (USA). Transcriptional expression was analyzed deploying String Tie (version 1.2.3) and quantified by FPKM (fragments per kilobase of exon per million fragments mapped). Differentially expressed genes (DEGs) were identified with an adjusted P value < 0.05 and a fold change > 1.5.
Western blotting analysis
Tissues and cells were lysed in RIPA buffer with fresh addition of 1% protease inhibitor cocktail (Bimake, Shanghai, China). The protein was quantified using the bicinchoninic acid method (Epizyme, shanghai, China). Protein (30–60 µg) was fractionated using 10% or 12.5% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred onto PVDF nitrocellulose membranes (Millipore, NY, USA). The membrane was blocked in 5% non-fat milk at room temperature for 1 h, washed with Tris buffered saline-Tween 20 (TBST) and then blotted with primary antibodies overnight at 4 °C. The second day, the membrane was washed with TBST three times and then incubated with corresponding secondary antibody for 1 h at room temperature. The antibodies used in this study were presented in Supplementary Table S4. The blots were then developed by chemiluminescence using Tanon ECL kits (Tanon, Shanghai, China). The gray values of protein bands were analyzed using Image J software.
Immunohistochemistry (IHC) staining
PFA fixed placental tissues were embedded in paraffin and 5 μm thick sections were cut (Runnerbio Technology CO., Ltd, Shanghai, China). Sections were dewaxed using xylene and dehydrated in an ethanol gradient. Endogenous peroxidase was quenched using 3% hydrogen peroxide (H2O2) in the dark for 20 min. The sections were blocked with 5% BSA at room temperature for 20 min and incubated with primary antibodies overnight at 4 °C. Next day, corresponding secondary antibody was added and incubated for 30 min at 37 °C. The sections were stained using 3, 3’-diaminobenzidine and hematoxylin and imaged under a light microscope.
Immunofluorescence (IF) staining
Cells were fixed with 4% PFA for 15 min at room temperature. After twice washing with PBS, cells were permeabilized with 0.25% Triton X-100 for 15 min, and then blocked by Immunol Staining Blocking Buffer (Beyotime, Shanghai, China) for 30 min. After that, cells were incubated with YTHDC1 primary antibody (1:200, NBP1-81353, NOVUS) overnight at 4 °C prior to incubation with fluorescence-labelled secondary antibody (1:200, R37120, Invitrogen) at room temperature for 1 h. Nuclei were stained with DAPI (Beyotime), and images were visualized using confocal microscopy.
RNA immunoprecipitation (RIP) and high-throughput sequencing (RIP-seq)
HTR-8/SVneo cells were lysed for 30 min on ice with IP lysis buffer containing 1 U/µl RNase inhibitor (Vazyme). The supernatant was harvested by centrifugating at 4 °C, 10,000 g for 15 min. The IP grade antibodies cross-linked with protein G beads were added to the lysate followed by incubation overnight at 4 °C. After sixth washing with NT2 buffer, the precipitated RNAs were extracted by Trizol reagent and analyzed by qRT-PCR. For sequencing, KAPA RNA HyperPrep Kit (Roche, USA) was employed to remove rRNAs, and cDNA libraries were generated using the NEBNext Ultra Directional RNA Library Prep kit (New England Biolabs, USA) and sequenced on the Illumina HiSeq 3000 platform. Each group was sequenced in duplicate.
Methylated RNA immunoprecipitation (MeRIP)-qPCR and MeRIP-seq
MeRIP-qPCR was performed using the riboMeRIP m6A Transcriptome Profiling Kit (RiboBio). In brief, total RNA was extracted and digested with DNase I before being divided into 100 bp-long fragments with RNA Fragmentation Buffer. Fragmented RNAs were then incubated with RNA beads on ice for 15 min. After ethanol precipitation twice, supernatants were collected. 5 µg m6A antibody (ab151230, Abcam) was pre-incubated with Protein G in IP buffer at room temperature for 1.5 h. Then, the fragmented RNA was incubated with m6A antibody-bead mixture at 4 °C overnight. qRT-PCR analysis and RNA-seq library construction were performed using the enriched RNA. MeRIP-seq and data analysis were supported by Epibiotek (Guangzhou, China). The library was prepared by the smart-seq method. Both the input samples and the m6A IP samples were subjected to 150-bp paired-end sequencing and sequenced on the Illumina NovaSeq 6000 platform.
Chromatin immunoprecipitation (ChIP) and ChIP-Seq
HTR-8/SVneo and JAR cells were treated with 1% PFA for crosslinking. Glycine was added to stop the reaction at a final concentration of 0.125 M. Cells were then scraped and lysed with ChIP lysis buffer containing a proteinase inhibitor. Sonication was then employed to shear the chromatin into 200–1000 bp fragments. Following this, IgG or ChIP grade antibodies were added to Protein A/G magnetic beads (Bimake) and incubated at room temperature for 60 min. The mixture was then added to the beads and incubated overnight at 4 °C with rotation. Next day, a magnetic field was used to remove nonspecific fragments, followed by fourth washing of the beads. Finally, the samples were eluted using MinElute Spin Columns (Qiagen, Hilden, Germany). The antibodies used in the ChIP assay were listed in Supplementary Table S4.
For library construction, a DNA-sequencing kit was employed, following the manufacturer’s instructions. TBE PAGE-gel size selection was performed to obtain ChIP-seq libraries with fragments ranging from 250 to 500 bp. For ChIP-seq data analyses, standard alignment parameters were used to align 150 bp paired-end reads to the reference human genome hg38 using Bowtie. For ChIP-seq analysis, Bam files were converted to BigWig files using deepTools, and peaks were identified using the MACS2 peak caller with the following parameters: -f BAMPE -keep-dup all -g hs -q 0.01. Peaks were annotated with Homer. IGV was used to visualize the distribution of identified peaks across genomic regions.
Surface sensing of translation (SUnSET) assay
Cells were incubated with 10 µg/ml puromycin for 15 min, followed by chasing for 60 min to confirm the efficiency of labeling. Cells were then lysed using RIPA lysis buffer (Epizyme), and analyzed by Western blotting using an anti-puromycin antibody (Millipore).
Nascent protein synthesis assay
De novo protein synthesis was detected using O-propargyl-puromycin (OPP) analysis. Briefly, JAR and HTR-8/SVneo cells were treated with OPP at 37 °C for 30 min before being fixed with 3.7% PFA for 15 min at room temperature. Following twice washing with PBS, cells were permeabilized for 15 min with 0.25% Triton X-100. Then, a Click-iT™ Plus OPP Alexa Fluor™ 488 Protein Synthesis Assay Kit (Thermo Fisher Scientific, USA) was used to detect fluorescent labelled nascent peptides incorporated with OPP.
Polysome profiling
JAR cells were pretreated with 100 µg/ml cycloheximide (CHX; Merck Millipore, Germany) for 5 min at 37 °C. Cells were then harvested and incubated with 800 µl polysome cell extraction buffer (10 mM NaCl, 10 mM MgCl2, 10 mM Tris-HCl (pH 7.5), 1% Triton X-100, 1% sodium deoxycholate, 0.2 U/µl RNase inhibitor, 1 mM DTT and 0.1 mg/ml cycloheximide). After centrifugation at 16,000 g at 4 °C for 10 min, the supernatants were harvested and loaded onto sucrose gradients ranging from 15 to 50%, followed by ultracentrifugation at 274,000 g in a Beckman SW41 rotor at 4 °C for 100 min. Samples were then fractionated by a density gradient fractionation system, and the absorbance at 254 nm was detected.
Protein stability assay
HTR-8/SVneo and JAR cells were treated with 50 µg/ml CHX, and harvested at the indicated time points (0, 12, 24, 36, 48 h). The protein levels of YTHDC1 and RPL37 were then determined by Western blotting analysis.
Dual-luciferase reporter assay
The promoter regions of YTHDC1 were amplified from genomic DNA of HTR-8/SVneo cells and cloned into the pGL3-basic reporter vector (Promega, USA). The primers were listed in Supplementary Table S3. Then, HEK-293T cells were seeded into 24-well plates at a density of 2 × 104 cells per well. Next day, 50 ng of pRL-TK, 500 ng of pGL3-basic or pGL3-YTHDC1-promoter, and 1.25 µl of siNC or RXRA siRNA were transfected into each well. Firefly and Renilla luciferase activities were measured using a dual-luciferase reporter assay system (Yeasen).
Coimmunoprecipitation (Co-IP)
Briefly, 1 × 107 HTR-8/SVneo cells were harvested and lysed with lysis buffer (2 mM MgCl2, 150 mM NaCl, 1% NP-40, 50 mM Tris-HCl (pH 7.5), 5% glycerol, 2 mM EDTA, and proteinase inhibitor) at 4 °C for 30 min. The supernatants were collected by centrifuging at 12,000 × g for 20 min. Next, 5 µg of the primary antibody or IgG control was added separately. Protein G beads were then added to the immune complexes after overnight incubation at 4 °C. Following 4 h of further incubation at 4 °C, the immune complexes were washed six times with NT2 buffer (150 mM NaCl, 1 mM MgCl2, 50 mM Tris-HCl (pH 7.5), and 0.05% NP-40) and boiled at 100 °C for 10 min. The YTHDC1-interacting proteins were identified by Western blotting analysis.
Statistical analysis
Unless otherwise specified, all data are presented as mean ± SEM and were analyzed using GraphPad Prism 8.0 software (GraphPad Inc., CA, USA). Each experiment was conducted at least in triplicate. The unpaired Student’s t-test was used to determine the statistical significance between two groups. For comparisons involving three or more groups, one-way or two-way ANOVA was employed. A P < 0.05 was considered statistically significant.
Results
YTHDC1 expression is elevated in human ART-preterm placentas
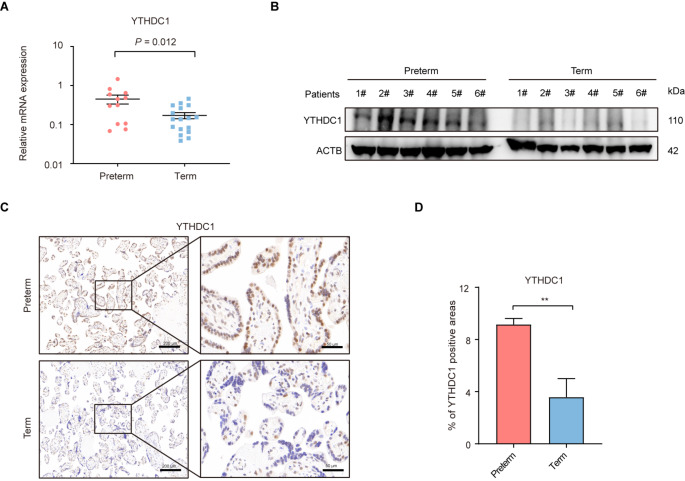
To determine whether m6A methylation participates in the pathogenesis of ART-preterm, qRT-PCR was initially employed to examine the mRNA levels of 27 m6A-related genes in human placentas. After strictly excluding complications (preeclampsia, gestational diabetes, gestational hypertension, and chorioamnionitis) related to PTB, the study finally included ART-preterm (n = 12) and ART-term (n = 17) placentas (Fig. 1A and Supplementary Figure S1A). We found that YTHDC1, EIF3A, IGF2BP3, and PRRC2A mRNA expression was significantly increased in ART-preterm placentas compared to that in ART-term placentas, whereas ALKBH5 mRNA expression was significantly decreased in ART-preterm placentas. Notably, YTHDC1 exhibited the most significant upregulation in the ART-preterm group, displaying a 2.63-fold increase (Fig. 1A). Consistently, Western blotting analysis further confirmed the enhanced expression of YTHDC1 in the placentas of the ART-preterm group (Fig. 1B). Subsequently, to explore the distribution of YTHDC1 in placental tissue, immunohistochemistry (IHC) was conducted. The results showed that YTHDC1 was distributed in both the cytotrophoblasts and syncytiotrophoblasts (STB), with the strongest staining observed in the STB (Fig. 1C, D).
Fig. 1.
YTHDC1 is upregulated in human ART-preterm placentas. (A) qRT-PCR detection of the expression level of YTHDC1 in placentas conceived by assisted reproductive technology (ART). Preterm: preterm placentas (n = 12); Term: full-term placentas (n = 17). (B) Western blotting detection of YTHDC1 in the placentas conceived by ART. Preterm (n = 6), Term (n = 6). (C, D) Immunohistochemistry (IHC) images and quantification of YTHDC1 positive staining areas in placentas conceived by ART. Preterm (n = 6), Term (n = 6). Scale bar, 200 μm and 50 μm. Data are shown as means ± SD. **P < 0.01
YTHDC1 promotes cell proliferation, migration, invasion, and inhibits cell apoptosis in trophoblastic cells
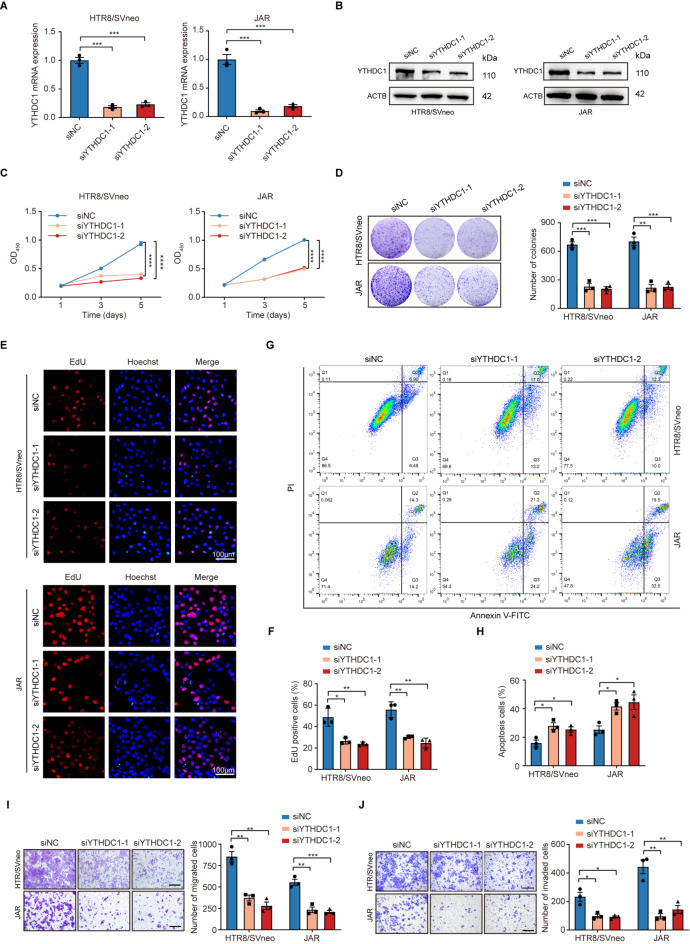
Previous studies have shown that abnormal proliferation and invasion capabilities of trophoblasts are involved in the pathogenesis of preterm delivery [28]. To explore the biological functions of YTHDC1 in trophoblastic cells, two small interfering RNAs (siRNAs) were synthesized to target endogenous YTHDC1 (Fig. 2A, B). Notably, YTHDC1 knockdown significantly decreased cell viability and colony formation ability in HTR8/SVneo and JAR cells (Fig. 2C, D). The EdU staining assay demonstrated that knockdown of YTHDC1 suppressed cell proliferation (Fig. 2E, F). Concurrently, an increased proportion of apoptotic cells was observed following YTHDC1 knockdown (Fig. 2G, H). Moreover, YTHDC1 knockdown impaired the migration and invasion capabilities, as indicated by the Transwell assays (Fig. 2I, J). We further generated YTHDC1-knockout trophoblastic cell lines deploying the CRISPR/Cas9 system and confirmed that YTHDC1 deficiency resulted in cell growth retardation and decrease of migration and invasion capabilities of HTR-8/SVneo and JAR cells (Supplementary Figure S2 A-F), whereas ectopic expression of YTHDC1 exhibited an opposite effect (Supplementary Figure S2 G-L). We further explored the biological functions of YTHDC1 in STB using PHT. Consistent with other in vitro results, knockdown of YTHDC1 remarkably promoted cellular senescence mediated by P21 and P16 and activated autophagy by Beclin-1 and LC3B (Supplementary Figure S3 A-C), while YTHDC1 overexpression played an opposite role. Furthermore, YTHDC1 could affect the expression of VEGF and sFLT-1, which are specially secreted by STB (Supplementary Figure S3 D). Collectively, these findings indicate that YTHDC1 plays a pivotal role in modulating trophoblastic function.
Fig. 2.
YTHDC1 knockdown inhibits the proliferation, migration, and invasion of trophoblastic cells. (A) Knockdown efficiency of YTHDC1 siRNA in HTR-8/SVneo and JAR cells as assessed by qRT-PCR. (B) Knockdown efficiency of YTHDC1 siRNA in HTR-8/SVneo and JAR cells as assessed by Western blotting. (C) Knockdown effects of YTHDC1 on the cellular viability of HTR-8/SVneo and JAR cells as detected by CCK-8. (D) Knockdown effects of YTHDC1 on the proliferation of HTR-8/SVneo and JAR cells as detected by colony formation assays. (E, F) Knockdown effects of YTHDC1 on the proliferation of HTR-8/SVneo and JAR cells as detected by EdU assay. Scale bar, 100 μm. (G, H) Cell apoptotic rate as analyzed by flow cytometry. (I, J) Knockdown effects of YTHDC1 on the migration (I) and invasion (J) of HTR-8/SVneo and JAR cells as detected by Transwell assay. Scale bar, 100 μm. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001
YTHDC1 modulates JAK/STAT signaling pathways in trophoblastic cells
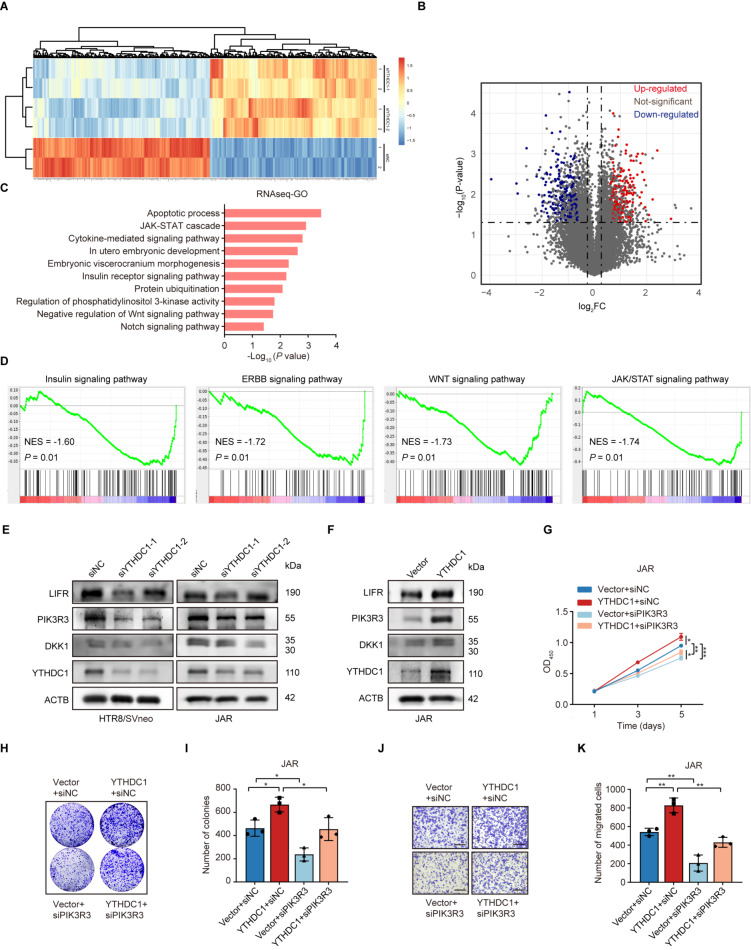
To explore the underlying signaling pathways regulated by YTHDC1, RNA-seq was performed in HTR-8/SVneo cells with YTHDC1 depletion (Fig. 3A). YTHDC1 knockdown resulted in 488 genes altered globally, including 237 up-regulated genes and 251 downregulated genes (Fig. 3B). Gene ontology (GO) analysis of downregulated (DEGs) revealed that several pathways, including the apoptotic process, JAK/STAT cascade, and WNT signaling pathways, were notably enriched (Fig. 3C). Gene set enrichment analysis (GSEA) also indicated that the genes modulated by YTHDC1 were associated with the JAK/STAT and WNT signaling pathways (Fig. 3D). Accumulating evidence indicates that there is abnormal activation of WNT and JAK/STAT signaling pathways in preterm placentas [29–31]. Therefore, the expression levels of top 12 DEGs (DKK1, FZD8, WNT7B, WNT5B, NKD2, JAK2, SOCS1, SOCS2, SOCS5, PIK3R3, LIFR, SOS1) related to the JAK/STAT and WNT signaling pathways were assessed. The results showed that PIK3R3, LIFR, and DKK1 were dramatically reduced upon YTHDC1 knockdown at both mRNA (Supplementary Figure S4) and protein levels (Fig. 3E) in HTR-8/SVneo and JAR cells, whereas overexpression of YTHDC1 showed an opposite effect (Fig. 3F). Notably, among these genes, PIK3R3 presented the most markedly expression change. Subsequently, rescue experiments were performed to investigate whether PIK3R3 participated in the biological functions of YTHDC1 in trophoblastic cells. As shown in Fig. 3G-K, PIK3R3 knockdown strikingly impaired the YTHDC1-mediated increase in cell proliferation and migration capabilities. Together, these data indicate that YTHDC1 can affect the JAK/STAT signaling pathway to modulate trophoblastic function.
Fig. 3.
Identification of the signaling pathways regulated by YTHDC1 in trophoblastic cells. (A) Heatmap of differentially expressed genes (DEGs) identified by RNA-seq in HTR-8/SVneo cells. (B) Volcano plot of DEGs. (C) GO enrichment analysis of DEGs. (D) GSEA analysis of DEGs. (E, F) The knockdown (E) and overexpression (F) effects of YTHDC1 on the expression of DKK1, PIK3R3, and LIFR in HTR-8/SVneo and JAR cells as detected by Western blotting. (G) CCK-8 assay of JAR cells transfected with a YTHDC1 overexpression plasmid and PIK3R3 siRNA. (H, I) Colony formation assay of JAR cells transfected with a YTHDC1 overexpression plasmid and PIK3R3 siRNA. (J, K) Transwell assay about migration of JAR cells transfected with a YTHDC1 overexpression plasmid and PIK3R3 siRNA. *P < 0.05, **P < 0.01, ***P < 0.001
YTHDC1 enhances mRNA translation and protein biosynthesis in trophoblastic cells
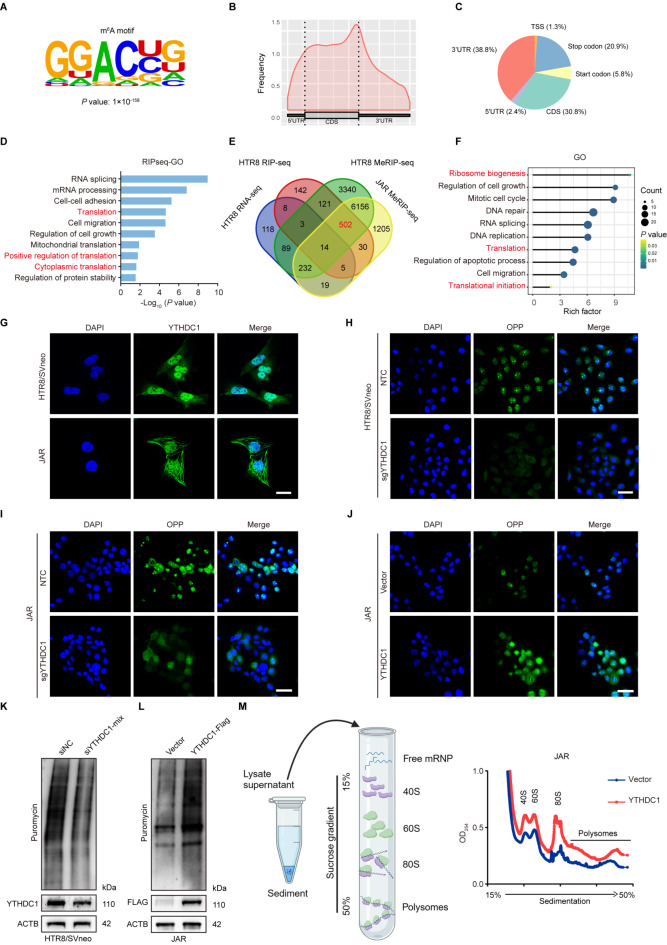
It is well-recognized that YTHDC1 acts as an m6A reader, and it functions via binding and affecting m6A-methylated transcript [32]. Thus, methylated RNA immunoprecipitation (MeRIP)-seq and YTHDC1 RIP-seq were performed in trophoblastic cells. MeRIP-seq identified 10,457 m6A-modified mRNAs in HTR-8/SVneo cells and 8,163 in JAR cells, respectively. MEME algorithm analysis identified the m6A consensus motif (GGAC), implying the successful enrichment of m6A-modified mRNAs (Fig. 4A). In accordance with reported studies [33], m6A peaks were predominantly enriched in 3′-untranslated regions (3′-UTR) (38.8%) and the coding sequence (CDS) (30.8%) (Fig. 4B, C). In addition, RIP-seq identified 825 transcripts specifically interacting with YTHDC1 (fold change ≥ 2, p < 0.05). Intriguingly, GO analysis demonstrated that YTHDC1-binding genes were mainly enriched in translation-related pathways (Fig. 4D). Next, an integrated approach combining MeRIP-seq, RIP-seq, and RNA-seq identified 516 potential targets of YTHDC1, among which 502 (97.2%) genes were not affected upon YTHDC1 knockdown (Fig. 4E). Subsequently, GO analysis illustrated that these 502 genes were involved in translation-related pathways and biological processes, including ribosome biogenesis, translation, and translation initiation process, indicating that YTHDC1 might participate in translational regulation (Fig. 4F).
Fig. 4.
YTHDC1 accelerates protein synthesis in trophoblastic cells. (A) The m6A motif detected by MEME algorithm analysis in identified mRNAs by MeRIP-seq in HTR-8/SVneo cells. (B) Metagene profiles of m6A enriched regions across mRNA segments in HTR-8/SVneo cells. (C) The distribution of m6A modified sites within mRNAs in HTR-8/SVneo cells. (D) GO enrichment analysis of the DEGs identified by RIP-seq. (E) Overlapping analysis of genes identified by RNA-seq, RIP-seq, and MeRIP-seq in HTR-8/SVneo and JAR cells. RNA-seq analysis was conducted on YTHDC1 knockdown HTR-8/SVneo cells. (F) Functional annotation of the overlapping genes. (G) IF staining of YTHDC1 in HTR-8/SVneo and JAR cells. Scale bar, 25 μm. (H-J) The effects of YTHDC1 knockout (H, I) or overexpression (J) on protein synthesis in HTR-8/SVneo and JAR cells detected by OP-Puro assays. Scale bar, 50 μm. (K, L) The effects of YTHDC1 knockdown (K) or overexpression (L) on de novo protein synthesis in HTR-8/SVneo and JAR cells as detected by SUnSET assays. (M) Polysome profiling of YTHDC1 overexpressed JAR cells
To test the above-mentioned hypothesis, immunofluorescence staining was conducted to examine the distribution of YTHDC1, as mRNA translation exclusively occurs in the cytoplasm. The results showed YTHDC1 presence in both the cytoplasm and nuclei of HTR-8/SVneo and JAR cells (Fig. 4G). Subsequently, OPP assays were performed to investigate the impact of YTHDC1 on de novo protein synthesis. We found that YTHDC1 deficiency markedly diminished de novo protein synthesis in both HTR-8/SVneo and JAR cells (Fig. 4H, I). Conversely, overexpression of YTHDC1 showed an opposite effect (Fig. 4J). To further strengthen our observations, the SUnSET approach was employed to monitor total cellular translation by detecting puromycin-labeled polypeptides. The results showed a notable reduction in total cellular translation upon YTHDC1 knockout compared to that in control groups (Fig. 4K), whereas YTHDC1 overexpression enhanced mRNA translation (Fig. 4L). Additionally, polysome profiling analysis revealed that YTHDC1 overexpression resulted in a remarkable increase in polysomes (Fig. 4M), indicating that YTHDC1 can fine-tune mRNA translation. To dissect the underlying mechanism, we performed the Co-IP assay and found that YTHDC1 interacted with a component of the eukaryotic translation initiation factor (eIF) complexes, namely eIF3A (Supplementary Figure S5 A). Collectively, all these data suggest that YTHDC1 might recruit eIF3A protein to regulate mRNA translation.
YTHDC1 modulates mRNA translation and protein biosynthesis by increasing RPL37 expression
Among the 502 genes identified from the overlap of MeRIP-seq and RIP-seq, 17 genes (RPL5, MRPS15, RPL36A-HNRNPH2, MRPL27, IGHMBP2, MRPS21, RRBP1, MRPL30, TNIP1, MTG1, RPL36AL, RPL24, RPL37, RPS24, ABCF1, EIF4G, RPL19) were predominantly enriched in the translation process. qRT-PCR analysis confirmed the upregulation of eIF4G and RPL37 in ART-preterm placentas (Fig. 5A). As expected, neither YTHDC1 knockdown nor overexpression affected the mRNA levels of eIF4G and RPL37 in both HTR-8/SVneo and JAR cells (Fig. 5B, C). However, Western blotting analysis showed that the protein level of RPL37 decreased dramatically in YTHDC1 knockdown cells and increased in YTHDC1 overexpression cells, whereas eIF4G level remained consistent (Fig. 5D, E). Since RPL37 protein level declined under YTHDC1 deficiency, we treated HTR8/SVneo and JAR cells with the protein translation inhibitor cycloheximide to exclude the possibility that YTHDC1 contributed to RPL37 protein stability. As shown in Supplementary Figure S5 C, D, YTHDC1 knockdown did not affect the protein stability of RPL37 in both cells, indicating that YTHDC1 regulated protein synthesis of RPL37.
Fig. 5.
YTHDC1 regulates RPL37 translation in an m6A-dependent manner. (A) Relative mRNA level of RPL37 and eIF4G in human ART-preterm and ART-term placentas as detected by qRT-PCR. Preterm: preterm placentas (n = 12); Term: full-term placentas (n = 17). (B, C) Relative mRNA level of RPL37 and eIF4G in HTR-8/SVneo and JAR cells upon YTHDC1 knockdown (B) or overexpression (C) as detected by qRT-PCR. (D, E) Relative protein levels of RPL37 and eIF4G in HTR-8/SVneo and JAR cells upon YTHDC1 knockdown (D) or overexpression (E) as detected by Western blotting. (F) Integrative genomics viewer (IGV) tracks of m6A peaks and YTHDC1 binding peaks across RPL37 transcript. (G) qRT-PCR analysis of RPL37 in the MeRIP products. MeRIP carried out in HTR-8/SVneo and JAR cells with antibody against m6A, or the control unimmunized IgG. (H) qRT-PCR analysis of RPL37 in the RIP products. RIP carried out in HTR-8/SVneo and JAR cells with antibody against YTHDC1, or the control unimmunized IgG. (I, J) Schematic representation of wild-type (RPL37-WT) and mutant (RPL37-MUT) RPL37 luciferase reporters. (K) Luciferase activities of RPL37-WT or RPL37-MUT measured in 293T cells with or without YTHDC1 knockout. (L) Western blotting detection of YTHDC1 and RPL37. HTR-8/SVneo and JAR cells were co-transfected with YTHDC1 siRNA and RPL37 overexpression plasmid. (M, N) Rescue assay of cellular viability as detected with CCK-8. HTR-8/SVneo (M) and JAR cells (N) were co-transfected with YTHDC1 siRNA and RPL37 overexpression plasmid. (O, P) Rescue assay of colony formation. (Q) Rescue assay of SUnSET experiment. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001
Subsequently, integrative genome viewer (IGV) analysis revealed that the m6A modification accumulated across the RPL37 transcript, and the m6A peaks coincided well with YTHDC1 binding sites (Fig. 5F). Further, MeRIP-qPCR (Fig. 5G) and RIP assays (Fig. 5H and Supplementary Figure S5 B) affirmed the presence of m6A modification and binding of YTHDC1 to RPL37 mRNA. Importantly, either depletion of METTL3 or overexpression of FTO decreased RPL37 protein levels in trophoblastic cells (Supplementary Figure S6 A, B). These data indicated that RPL37 mRNA was modified by m6A. To explore whether YTHDC1-induced translation progression is dependent on m6A-modified RPL37 mRNA, we inserted the wild-type (WT) RPL37-3′UTR sequence or a mutant containing the mutated putative m6A site (RPL37-MUT) into a firefly luciferase reporter (Fig. 5I, J). Subsequently, luciferase measurement indicated the activity of RPL37-WT reporter was significantly decreased upon YTHDC1 depletion, but the RPL37-MUT group was resistant to the effect of YTHDC1 knockout (Fig. 5K). In addition, knockdown of METTL3 or overexpression of FTO markedly abolished the increased protein level of RPL37 induced by YTHDC1 overexpression (Supplementary Figure S6 C, D). Collectively, these data suggest that YTHDC1 directly binds to RPL37 mRNA and augments RPL37 expression in an m6A-dependent manner.
RPL37 is an essential part of the ribosomal 60 S subunit. Western blotting analysis revealed a remarkably higher RPL37 level in human ART-preterm placentas than in ART-term placentas (Supplementary Figure S6 E). Therefore, the biological functions of RPL37 in trophoblastic cells were investigated. Both CCK-8 and colony formation assays illustrated that RPL37 knockdown inhibited cell proliferation in HTR-8/SVneo and JAR cells. Consistently, RPL37 knockdown decreased the migration and invasion capacities of trophoblastic cells (Supplementary Figure S6 F-J). Moreover, ectopic expression of RPL37 attenuated the decreases in cell viability and colony formation ability in YTHDC1 knockdown cells (Fig. 5L-P). Accordingly, de novo protein biosynthesis suppressed by YTHDC1 loss was re-established after RPL37 overexpression (Fig. 5Q), indicating that RPL37 is a functional target of YTHDC1 in trophoblastic cells.
To explore the relationship of YTHDC1, RPL37 and JAK/STAT signaling pathway, we initially examined the status of m6A modification across PIK3R3 transcript by MeRIP-qPCR assay. The findings showed that neither METTL3 depletion nor FTO overexpression affected the level of immunoprecipitated PIK3R3 mRNA, suggesting that PIK3R3 mRNA was not modified by m6A. In contrast, silencing of METTL3 or ectopic expression of FTO dramatically dampened the m6A modification status in RPL37 mRNA (Supplementary Figure S7 A, B). Meanwhile, Western blotting assay showed that RPL37 overexpression significantly attenuated the effect of YTHDC1 knockdown on PIK3R3 protein expression. Moreover, RPL37 depletion markedly impaired the increased PIK3R3 protein level induced by YTHDC1 overexpression (Supplementary Figure S7 C, D). Collectively, these results indicate that YTHDC1 fine-tuning the JAK/STAT signaling pathway is mediated by RPL37.
RXRA is required for the activation of YTHDC1 transcription
To investigate the molecular mechanism driving YTHDC1 expression in trophoblastic cells, we explored the possible transcription factors for YTHDC1. Luciferase reporter assay of a series of YTHDC1 promoter truncations showed that the region of -300~ -400 bp upstream from the transcription start site (TSS) was the core promoter region (Supplementary Figure S8 A; Fig. 6A). The possible transcription factors in the -300~ -400 bp region were predicted deploying PROMO software (https://alggen.lsi.upc.es/cgi-bin/promo_v3/promo/promoinit.cgi?dirDB=TF_8.3). The results showed that 14 potential transcription factors may be involved in YTHDC1 transcriptional regulation (Supplementary Figure S8 B). Among them, RXRA and GTF2I were upregulated in ART-preterm placental tissues compared to corresponding controls (Fig. 6B). An siRNA screening assay demonstrated that RXRA knockdown significantly reduced YTHDC1 expression at both the mRNA and protein levels (Fig. 6C, D). A luciferase reporter assay also validated that knockdown of RXRA inhibited YTHDC1 promoter activity, whereas RXRA overexpression showed an opposite effect (Fig. 6E). Moreover, the ChIP assay exhibited that RXRA was significantly enriched in the promoter region of YTHDC1 (Fig. 6F, G), indicating that RXRA could activate YTHDC1 transcription. The ART process involves a high risk of estrogen exposure, and this high estrogen status can persist through whole early pregnancy [13]. To investigate the potential influence of high estradiol (E2) exposure on YTHDC1 expression, HTR-8/SVneo and JAR cells were treated with E2. Western blotting analysis showed that E2 exposure significantly increased the protein levels of YTHDC1. Intriguingly, the protein levels of RXRA and RPL37 were also upregulated post E2 exposure (Fig. 6H). Collectively, these data suggest that E2 may be involved in the transcriptional regulation of YTHDC1 by RXRA.
Fig. 6.
Estradiol (E2) promotes YTHDC1 transcription through RXRA. (A) Luciferase assay of the YTHDC1 promoter. HEK-293T cells were transfected with reporter plasmids containing a series of YTHDC1 promoter truncations. (B) qRT-PCR quantification of potential transcription factors, RXRA and GTF2I, in human placental tissues. The potential transcription factors about the human YTHDC1 promoter between − 1000 to -900 bp are predicted by the ALGGEN-PROMO version 8.3 online tool of TRANSFAC. (C) Relative mRNA level of YTHDC1 as detected by qRT-PCR in HTR-8/SVneo and JAR cells upon knockdown of RXRA or GTF2I. (D) Western blotting analysis of YTHDC1 in HTR-8/SVneo and JAR cells upon knockdown of RXRA or GTF2I. (E) Luciferase assay about the effect of RXRA on the transcription of YTHDC1. HEK-293T cells were transfected with RXRA siRNA or overexpression plasmid. (F) Alignment of ChIP-seq data from HTR-8/SVneo to the hg38 genome. The box showed the enrichment of RXRA and H3K27ac in the promoter of YTHDC1. (G) ChIP-qPCR assay about the binding of RXRA to the promoter of YTHDC1 in HTR-8/SVneo and JAR cells. (H) Western blotting analysis of RXRA, YTHDC1, RPL37. HTR-8/SVneo and JAR cells were treated with E2 at the indicated concentrations for 24 h. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001
siYTHDC1 administration effectively postpones murine preterm delivery
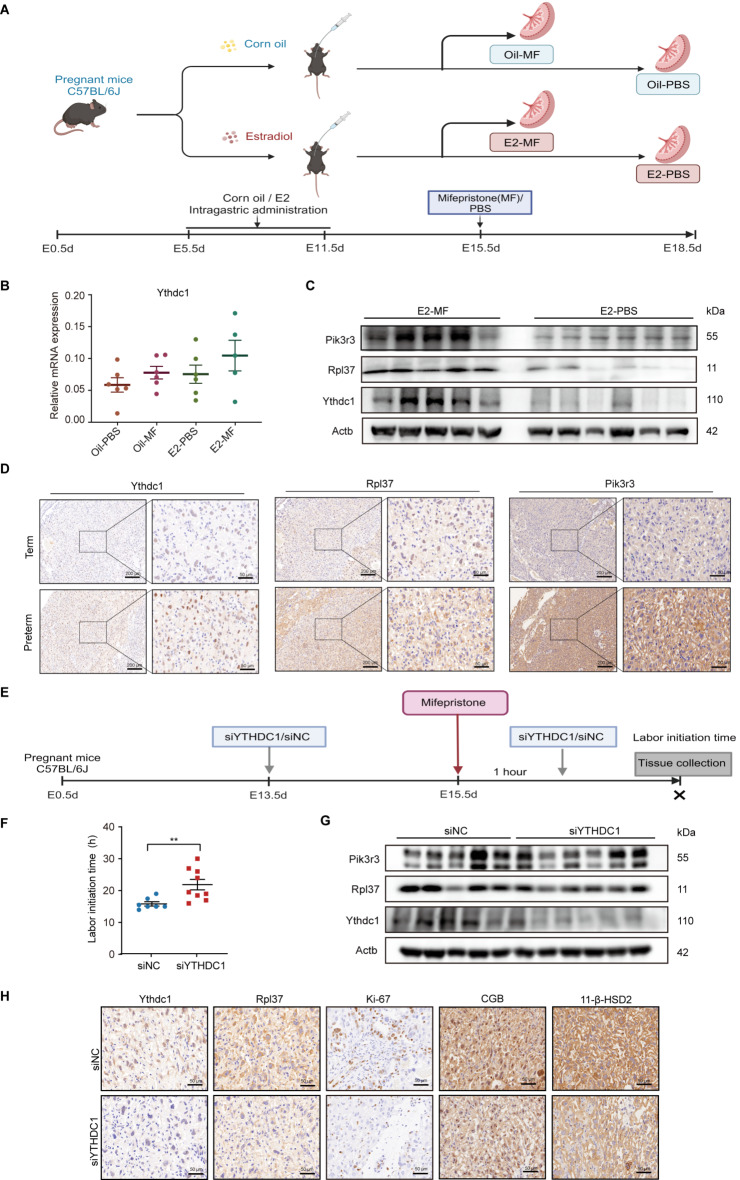
Our recent study has shown that individuals receiving artificial cycle of frozen embryo transfer (AC-FET) are more prone to give preterm birth (Supplementary Table S5) [34]. Importantly, AC-FET patients always receive treatment with exogenous estrogen, which prompted us to speculate that high E2 exposure might be involved in the pathogenesis of preterm delivery. To support the hypothesis, a mouse model under high E2 exposure was established. Initially, pregnant C57BL/6J mice were pretreated with E2 during early pregnancy, after which preterm delivery was initiated deploying RU-486, an antagonist for progesterone and glucocorticoid receptors (Fig. 7A). The results showed that high E2 exposure significantly increased the expression levels of YTHDC1, RPL37, and PIK3R3 in the placentas of E2-MF mice (Fig. 7B, C). These observations were further validated by IHC staining (Fig. 7D).
Fig. 7.
High E2 exposure elevates YTHDC1 expression and knockdown of YTHDC1 postpones murine preterm delivery in vivo. (A-D) Murine model of high E2 exposure. C57BL/6J mice were pretreated with E2 or corn oil, and preterm delivery was induced at E15.5d by RU486, while controls were treated with PBS (Oil-MF, n = 6; Oil-PBS, n = 6, E2-MF, n = 5; E2-PBS, n = 6). (A) The schematic diagram. (B) Relative mRNA level of YTHDC1 in the placentas as detected by qRT-PCR. (C) Western blotting detection of the expression of YTHDC1, RPL37, and PIK3R3 in the placentas. (D) Representative IHC staining images of YTHDC1, RPL37 and PIK3R3 in the placentas. Scale bar, 200 μm and 50 μm. (E-H) The schematic diagram for murine model of YTHDC1 siRNA treatment. Pregnant C57BL/6J mice were was intravenously administered with siYTHDC1 or siNC via the tail vein, and preterm delivery was induced at E15.5d by RU486. (E) The schematic diagram. (F) The initiation time of preterm labor (siYTHDC1, n = 9; siNC, n = 7). (G) Western blotting detection of YTHDC1, RPL37, and PIK3R3 in the placentas. (H) Representative IHC staining images of YTHDC1, RPL37, Ki-67, 11-β-HSD2 and CGB in the placentas. Scale bar, 50 μm. **P < 0.01
Next, to determine the role of YTHDC1 in preterm delivery in vivo, siYTHDC1 was administered intravenously to pregnant C57BL/6J mice via the tail vein, concurrent with RU486 (Fig. 7E). Our data showed that pregnant mice administered with siYTHDC1 exhibited significantly delayed preterm labor compared to the control group. The average onset time for preterm labor was 21.89 ± 4.97 h in the YTHDC1-silencing group, in contrast to 15.86 ± 1.75 h in the control group (Fig. 7F). As expected, the protein levels of YTHDC1, RPL37, and PIK3R3 were strikingly diminished post administration of siYTHDC1 (Fig. 7G). These observations were further validated by IHC staining (Fig. 7H). Accordingly, the cell proliferation marker Ki67 was lower in the YTHDC1-silencing group (Fig. 7H). To determine the role of YTHDC1 in STB in vivo, the expression of markers of the STB, 11-β-HSD2 and CGB, were also examined by IHC (Fig. 7H). The results suggested that knockdown of YTHDC1 decreased 11-β-HSD2 and CGB levels. Taken together, these findings indicate that siYTHDC1 administration is a promising therapeutic approach for targeting preterm delivery.
Discussion
ART is an effective method of treating infertility, resulting in more than 8 million births worldwide so far [35]. However, deliveries resulting from ART pregnancies are at a higher risk PTB than those from natural conceptions, even in singleton pregnancy. Promising researches have enhanced the prognosis and treatment of PTB [4, 9, 28]. Nevertheless, the underlying molecular pathophysiology of PTB remains largely unknown. The multifactorial nature of PTB makes the identification of causal pathways contributing to it an arduous task. Infection and inflammation, stress, uterine distension, and placental abnormalities have all been identified as potential contributors to the etiology of PTB [4]. Although previous research has indicated that PTB is typically associated with events occurring at the late gestational stage, recent studies have proposed that abnormalities in embryo implantation and early placentation also play a critical role [36, 37].
Epigenetic regulation, especially m6A modification, plays a pivotal role in the development and function of the placenta [14, 38]. In this study, we found that the m6A reader YTHDC1 was upregulated in preterm placentas compared to full-term placentas in the ART population. Additionally, in vitro and vivo evidences suggest that increased YTHDC1 level enhances proliferation, migration, and invasion of trophoblastic cells. Trophoblastic dysfunction is closely related to PTB [39]. A number of studies have shown that insufficient placental proliferation and invasion are the main mechanisms underlying PTB [40, 41]. Nonetheless, other studies have suggested that excessive trophoblastic invasion is also involved in the pathogenesis of PTB [36]. Therefore, homeostasis of proliferation, migration, and invasion of trophoblastic cells in the placenta is required during pregnancy. This study found that YTHDC1 could accelerate cell proliferation, migration, and invasion, and inhibit cell apoptosis in trophoblastic cells. In particular, it could inhibit autophagy and senescence in STB. Crucially, in vivo siRNA targeting of YTHDC1 effectively delayed preterm birth. Therefore, these results suggest that YTHDC1 is a key molecule in preterm delivery.
The ART process involves a variety of adverse exposures. Clinically, the use of exogenous estrogen to enhance endometrial thickness in ART patients is common [42]. Studies have indicated that the utilization of exogenous estrogen can lead to elevated serum E2 levels [13]. Elevated maternal serum E2 has been linked to an increased risk of low birth weight, thyroid dysfunction, and abnormal lipid metabolism [13, 43]. The findings in our cohort suggested AC-FET with the use of exogenous estrogen was a risk factor for PTB, which is in line with the results of Zaat et al. [44]. In this study, we observed an upregulation of YTHDC1 in ART preterm placentas, but not in natural pregnancies (Supplementary Figure S1 B). We also demonstrated that E2 treatment increased YTHDC1 expression in trophoblastic cells, both in vitro and in vivo. The etiology of preterm delivery is complex and multifactorial. High estrogen exposure alone does not directly lead to PTB, as shown in our animal model of high estrogen exposure, but increases the susceptibility to PTB. Hence, our findings hint that excessive estrogen exposure during ART could be a significant factor in premature delivery.
Studies have shown that m6A modification and its associated regulators play pivotal roles in the development and function of trophoblastic cells [45]. For instance, m6A modification of lncRNA HZ01 can inhibit human trophoblastic cell proliferation and trigger miscarriage [23]. In preeclampsia, ALKBH5 regulates the function of trophoblastic cells by targeting CYR61 [46]. However, the functional and regulatory mechanisms of YTHDC1, particularly in trophoblastic cells, are still largely uncharted. Past research has indicated that YTHDC1 modulates leukemogenesis through the regulation of DNA replication determinant, MCM4 [47]. In renal cancer cells, YTHDC1 has been found to downregulate the ANXA1/MAPK pathway [32]. In this study, we found that YTHDC1 modulated the JAK/STAT and WNT signaling pathways. The crucial roles of these pathways in supporting trophoblastic proliferation, migration, and invasion have been validated [48, 49]. Additionally, PIK3R3, an essential regulatory subunit of PI3K, was found to be modulated by YTHDC1. It has been reported that PIK3R3 can enhance cellular proliferation, migration, and invasion [50], counteracting cell apoptosis and influencing inflammation, angiogenesis, and immune response [51, 52]. We present primary evidences that YTHDC1 can regulate PIK3R3 expression in trophoblastic cells, suggesting a promising avenue for further research into PIK3R3 expression and its regulatory mechanism concerning gestational disorders.
Many studies have unveiled abnormalities in translational regulation as common occurrences in human diseases [53, 54]. Our study reveals a previously unrecognized function of YTHDC1 in promoting proliferation and invasion by mediating translational progress. The results support that YTHDC1 is not only an endonuclear protein, but also a cytoplasmic protein. Our findings add to the understanding of the biological function of YTHDC1. In addition, we identified that YTHDC1 directly bound to m6A-modified RPL37 mRNA and regulated protein synthesis. Previous works on RPL37 have primarily centered on its role in the structural assembly process. Limited studies exist regarding RPL37’s function in disease development. We discovered that RPL37 possessed m6A modification and was regulated by YTHDC1. In addition, RPL37 was involved in YTHDC1-mediated JAK/STAT signaling pathway. Significantly, RPL37 overexpression mitigated the effects of YTHDC1 depletion on trophoblastic cell proliferation. These findings enrich our understanding about RPL37’s crucial roles and novel molecular functions. RPL37 might emerge as another prediction and therapeutic target for preterm delivery. However, the observed difference in RPL37 mRNA levels between human ART-preterm and ART-term placental tissues indicate that RPL37’s regulation is not solely via m6A modification, suggesting other potential regulatory mechanisms.
To date, only a few studies have identified transcription factors for YTHDC1. Recent research showed that YTHDC1 was regulated by the YY1/HDAC2 complex in renal cell carcinoma [32], while another study suggested that ZFP36 promoted YTHDC1 mRNA degradation in activated macrophages [55]. In this work, we demonstrated that E2 enhanced YTHDC1 expression via RXRA, while RXRA expression levels was higher in ART preterm placentas than in full-term ones. As a member of the nuclear receptor superfamily, RXRA is phylogenetically highly conserved. It is implicated in numerous biological processes, such as cell differentiation, senescence, and lipid metabolism [56]. Nonetheless, the precise mechanism by which E2 modulates RXRA expression to influence YTHDC1 expression in trophoblastic cells warrants further investigation.
Collectively, this study reveals that abnormal YTHDC1 expression results in aberrant trophoblastic proliferation and invasion, thereby contributing to preterm delivery of pregnancies conceived by ART. Mechanistically, E2 enhances YTHDC1 expression via the transcriptional factor RXRA. YTHDC1 promotes the translation of RPL37 in an m6A-dependent manner and can indirectly affect the expression levels of specific proteins in the WNT and JAK/STAT signaling pathways (Fig. 8). The elucidated E2/RXRA/YTHDC1/RPL37 axis enriches our understanding of the molecular mechanism underlying preterm pathogenesis. Additionally, this study offers a potential therapeutic target for preterm delivery.
Fig. 8.
The schematic diagram about working model of YTHDC1. Estradiol (E2) promoted YTHDC1 expression through RXRA upregulation. Elevated YTHDC1 upregulated the expression of RPL37 by promoting the binding of YTHDC1 to m6A-modified RPL37 mRNA, thereby augmenting total mRNA translation and indirectly modulated the JAK/STAT/PIK3R3 signaling pathway in trophoblastic cells
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors appreciate Dr. Junjiao Song (Shanghai Cancer Institute, Fudan University) for her guidance and assistance in mRNA translation and data analysis.
Author contributions
Z.L. and J.Z. initiated, conceived, and supervised the study. W.L., J.Z., and Z.L. were involved in the study design. All the authors who contributed to this work approved the final version of the manuscript. W.L., J.Z., B.L., Z.C., Z.L., C.C., and Q.S. performed the in vitro experiments. W.L., M.N., B.L., Z.C., D.Y., X.D., and H.S. were responsible for the in vivo experiments. W.L., Q.Z., M.N. X.L., Z.T. and X.H. performed statistical analyses. W.L. and J.Z. prepared the manuscript. W.L., J.Z., and Z.L. proofread the manuscript and take responsibility for the integrity of the data. All authors critically reviewed and provided feedback on the drafts and approved the final version.
Funding
This work was supported by the National Key R&D Program of China (2022YFC2702903), the National Natural Science Foundation of China Grants (81974232, 82271742), the Clinical Research Plan of SHDC (SHDC2020CR6027, SHDC22022303), the Program of Shanghai Academic Research Leader (21XD1403700), the Interdisciplinary Program of Shanghai Jiao Tong University (YG2021ZD29), the Shanghai Municipal Science and Technology Major Project (20Z11900602, 23DZ1203006), and the Medical Science and Technology Talents Support Project of IPMCH (Honghu Plan-HHJH2406).
Data availability
The sequencing data that support the findings of this study are listed in NCBI Gene Expression Omnibus (Accession no: GSE244402). Other data that support the findings of this study are available from the corresponding author upon reasonable request.
Declarations
Ethics committee approval and patient consent
The collection of human placentas was authorized by the Medical Ethics Committee of the International Peace Maternity and Child Health Hospital, affiliated with Shanghai Jiao Tong University School of Medicine (approval number: GKLW-2016-21). All animal experiments received approval from the Shanghai Jiao Tong University School of Medicine Institutional Animal Care & Use Committee (approval number: B-2022-015) and adhered to the approved guidelines.
Competing interests
The authors declared that they have no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Jiuru Zhao, Email: zhaojiurv@163.com.
Zhiwei Liu, Email: liuzhiwei@hotmail.com.
References
- 1.Yang XK, Li Y, Li CD, Zhang WY (2014) Current overview of pregnancy complications and live-birth outcome of assisted reproductive technology in mainland China. Fertil Steril 101(2):385–91 [DOI] [PubMed] [Google Scholar]
- 2.Barbuscia A, Martikainen P, Myrskylä M, Remes H, Somigliana E, Klemetti R, Goisis A (2020) Maternal age and risk of low birth weight and premature birth in children conceived through medically assisted reproduction. Evidence from Finnish population registers. Hum Reprod 35(1):212–220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cui LL, Zhou W, Xi B, Ma JL, Hu JM, Fang M, Hu KN, Qin YY, You L, Cao YZ et al (2020) Increased risk of metabolic dysfunction in children conceived by assisted reproductive technology. Diabetologia 63(10):2150–2157 [DOI] [PubMed] [Google Scholar]
- 4.Herrera CL, Maiti K, Smith R (2022) Preterm Birth and Corticotrophin-releasing hormone as a placental clock. Endocrinology 164(2) [DOI] [PMC free article] [PubMed]
- 5.Sunderam S, Kissin DM, Zhang YJ, Jewett A, Boulet SL, Warner L, Kroelinger CD, Barfield WD (2022) Assisted Reproductive Technology Surveillance - United States, 2018. Mmwr Surveill Summ 71(4):1–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mani S, Ghosh J, Lan YM, Senapati S, Ord T, Sapienza C, Coutifaris C, Mainigi M (2019) Epigenetic changes in preterm birth placenta suggest a role for ADAMTS genes in spontaneous preterm birth. Hum Mol Genet 28(1):84–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brosens I, Pijnenborg R, Vercruysse L, Romero R (2011) The great obstetrical syndromes are associated with disorders of deep placentation. Am J Obstet Gynecol 204(3):193–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Prins JR, Schoots MH, Wessels JI, Campmans-Kuijpers MJE, Navis GJ, van Goor H, Robertson SA, van der Beek EM, Sobrevia L, Gordijn SJ (2022) The influence of the dietary exposome on oxidative stress in pregnancy complications. Mol Aspects Med 87:101098 [DOI] [PubMed]
- 9.Morgan TK (2016) Role of the Placenta in Preterm Birth: a review. Am J Perinat 33(3):258–266 [DOI] [PubMed] [Google Scholar]
- 10.Eggenhuizen GM, Go A, Koster MPH, Baart EB, Galjaard RJ (2021) Confined placental mosaicism and the association with pregnancy outcome and fetal growth: a review of the literature. Hum Reprod Update 27(5):885–903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ren HY, Li YH, Jiang H, Du MQ (2016) Interferon-Gamma and Fas are involved in Porphyromonas gingivalis-Induced apoptosis of human extravillous trophoblast-derived HTR8/SVneo cells via Extracellular Signal-regulated kinase 1/2 pathway. J Periodontol 87(11):E192–E199 [DOI] [PubMed] [Google Scholar]
- 12.Nelissen ECM, Dumoulin JCM, Busato F, Ponger L, Eijssen LM, Evers JLH, Tost J, van Montfoort APA (2014) Altered gene expression in human placentas after IVF/ICSI. Hum Reprod 29(12):2821–2831 [DOI] [PubMed] [Google Scholar]
- 13.Hu XL, Feng C, Lin XH, Zhong ZX, Zhu YM, Lv PP, Lv M, Meng Y, Zhang D, Lu XE et al (2014) High maternal serum estradiol environment in the First Trimester is Associated with the increased risk of small-for-gestational-age birth. J Clin Endocr Metab 99(6):2217–2224 [DOI] [PubMed] [Google Scholar]
- 14.Nelissen EC, van Montfoort AP, Dumoulin JC, Evers JL (2011) Epigenetics and the placenta. Hum Reprod Update 17(3):397–417 [DOI] [PubMed] [Google Scholar]
- 15.Jiang XL, Liu BY, Nie Z, Duan LC, Xiong QX, Jin ZX, Yang CP, Chen YB (2021) The role of m6A modification in the biological functions and diseases. Signal Transduct Tar 6(1) [DOI] [PMC free article] [PubMed]
- 16.Wang X, Ma R, Zhang XL, Cui L, Ding YF, Shi WM, Guo CY, Shi YL (2021) Crosstalk between N6-methyladenosine modification and circular RNAs: current understanding and future directions. Mol Cancer 20(1) [DOI] [PMC free article] [PubMed]
- 17.Roundtree IA, Evans ME, Pan T, He C (2017) Dynamic RNA modifications in Gene expression regulation. Cell 169(7):1187–1200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang X, Lu ZK, Gomez A, Hon GC, Yue YN, Han DL, Fu Y, Parisien M, Dai Q, Jia GF et al (2014) N-6-methyladenosine-dependent regulation of messenger RNA stability. Nature 505(7481):117– [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang X, Zhao BS, Roundtree IA, Lu ZK, Han DL, Ma HH, Weng XC, Chen K, Shi HL, He C (2015) N-6-methyladenosine modulates Messenger RNA translation efficiency. Cell 161(6):1388–1399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guo X, Gao C, Yang DH, Li S (2023) Exosomal circular RNAs: a chief culprit in cancer chemotherapy resistance. Drug Resist Updat 67:100937 [DOI] [PubMed] [Google Scholar]
- 21.Chen FX, Wang QQ, Zhou YF (2021) The construction and validation of an RNA binding protein-related prognostic model for bladder cancer. BMC Cancer 21(1) [DOI] [PMC free article] [PubMed]
- 22.Li R, Qiu X, He M, Qiao J, He J, Zhong M (2022) METTL3-mediated mature miR-497-5p/195-5p inhibits trophoblast migration and invasion by targeting WWP1 in preeclampsia. Cell Cycle 21(13):Iii–Xviii [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 23.Xu ZY, Tian P, Guo JR, Mi CY, Liang TT, Xie JY, Huang WX, Dai MY, Chen WN, Zhang HD (2021) Lnc-HZ01 with m6A RNA methylation inhibits human trophoblast cell proliferation and induces miscarriage by up-regulating BPDE-activated lnc-HZ01/MXD1 positive feedback loop. Sci Total Environ 776:145950 [DOI] [PubMed]
- 24.Kliman HJ, Nestler JE, Sermasi E, Sanger JM, Strauss JF 3 (1986) Purification, characterization, and in vitro differentiation of cytotrophoblasts from human term placentae. Endocrinology 118(4):1567–1582 [DOI] [PubMed]
- 25.Li L, Schust DJ (2015) Isolation, purification and in vitro differentiation of cytotrophoblast cells from human term placenta. Reprod Biol Endocrinol 13:71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee B, Kroener LL, Xu N, Wang ET, Banks A, Williams J 3rd, Goodarzi MO, Chen YI, Tang J, Wang Y et al (2016) Function and hormonal regulation of GATA3 in Human First Trimester Placentation. Biol Reprod 95(5):113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.He WH, Jin MM, Liu AP, Zhou Y, Hu XL, Zhu YM, Liu AX (2019) Estradiol promotes trophoblast viability and invasion by activating SGK1. Biomed Pharmacother 117:109092 [DOI] [PubMed] [Google Scholar]
- 28.McNally L, Zhou Y, Robinson JF, Zhao GF, Chen LM, Chen H, Kim MY, Kapidzic M, Gormley M, Hannibal R et al (2020) Up-regulated cytotrophoblast DOCK4 contributes to over-invasion in placenta accreta spectrum. P Natl Acad Sci USA 117(27):15852–15861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Akram KM, Kulkarni NS, Brook A, Wyles MD, Anumba DOC (2022) Transcriptomic analysis of the human placenta reveals trophoblast dysfunction and augmented wnt signalling associated with spontaneous preterm birth. Front Cell Dev Biol 10:987740 [DOI] [PMC free article] [PubMed]
- 30.Chen XH, Tong C, Li HY, Peng W, Li R, Luo X, Ge HS, Ran YX, Li Q, Liu YM et al (2018) Dysregulated expression of RPS4Y1 (ribosomal protein S4, Y-Linked 1) impairs STAT3 (Signal Transducer and activator of transcription 3) signaling to suppress Trophoblast Cell Migration and Invasion in Preeclampsia. Hypertension 71(3):481–490 [DOI] [PubMed] [Google Scholar]
- 31.Deng QF, Wan QY, Liao J, Fang DR, Wang LL, Xiong SM, Xu P, Shen XB, Li Q, Zhou YZ (2022) Nickel nanoparticles affect the migration and invasion of HTR-8/SVneo cells by downregulating MMP2 through the PI3K/AKT pathway. Toxicol Vitro 80:105328 [DOI] [PubMed]
- 32.Li W, Ye K, Li XR, Liu XL, Peng M, Chen F, Xiong W, Wang YH, Zhu L (2022) YTHDC1 is downregulated by the YY1/HDAC2 complex and controls the sensitivity of ccRCC to sunitinib by targeting the ANXA1-MAPK pathway. J Exp Clin Canc Res 41(1) [DOI] [PMC free article] [PubMed]
- 33.Liu T, Wei QL, Jin J, Luo QY, Liu Y, Yang Y, Cheng CM, Li LF, Pi JN, Si YM et al (2020) The m(6)a reader YTHDF1 promotes ovarian cancer progression via augmenting EIF3C translation. Nucleic Acids Res 48(7):3816–3831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li W, Zhao JR, Ni M, Zhang QQ, Shen QW, Li H, Tang Z, Yao DT, Wang T, Qi SD et al (2023) Assisted reproductive technology and neurodevelopmental outcomes in offspring: a prospective birth cohort study in East China. Reprod Biomed Online 46(6):983–994 [DOI] [PubMed] [Google Scholar]
- 35.Elhakeem A, Taylor AE, Inskip HM, Huang J, Tafflet M, Vinther JL, Asta F, Erkamp JS, Gagliardi L, Guerlich K et al (2022) Association of assisted Reproductive Technology with offspring growth and adiposity from infancy to early adulthood. Jama Netw Open 5(7) [DOI] [PMC free article] [PubMed]
- 36.McNally L, Zhou Y, Robinson JF, Zhao G, Chen LM, Chen H, Kim MY, Kapidzic M, Gormley M, Hannibal R et al (2020) Up-regulated cytotrophoblast DOCK4 contributes to over-invasion in placenta accreta spectrum. Proc Natl Acad Sci U S A 117(27):15852–15861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Institute of Medicine Committee on Understanding, Premature B, Assuring Healthy O (2007) The National Academies Collection: Reports funded by National Institutes of Health. In: Preterm Birth: Causes, Consequences, and Prevention. edn. Edited by Behrman RE, Butler AS. Washington (DC): National Academies Press (US) Copyright © 2007, National Academy of Sciences [PubMed]
- 38.Zhang C, Liu N (2022) N6-methyladenosine (m6A) modification in gynecological malignancies. J Cell Physiol 237(9):3465–3479 [DOI] [PubMed] [Google Scholar]
- 39.Lee HJ, Lim SM, Jang HY, Kim YR, Hong JS, Kim GJ (2021) Mir-373-3p regulates Invasion and Migration Abilities of Trophoblast Cells via targeted CD44 and Radixin. Int J Mol Sci 22(12) [DOI] [PMC free article] [PubMed]
- 40.Vidal MS Jr., Lintao RCV, Severino MEL, Tantengco OAG, Menon R (2022) Spontaneous preterm birth: involvement of multiple feto-maternal tissues and organ systems, differing mechanisms, and pathways. Front Endocrinol (Lausanne) 13:1015622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Morgan TK (2016) Role of the Placenta in Preterm Birth: a review. Am J Perinatol 33(3):258–266 [DOI] [PubMed] [Google Scholar]
- 42.Eftekhar M, Tabibnejad N, Tabatabaie AA (2018) The thin endometrium in assisted reproductive technology: an ongoing challenge. Middle East Fertil S 23(1):1–7 [Google Scholar]
- 43.Wang HH, Zhou CL, Lv M, Yang Q, Li JX, Hou M, Lin J, Liu XM, Wu YT, Sheng JZ et al (2018) Prenatal high estradiol exposure induces sex-specific and dietarily reversible insulin resistance through decreased hypothalamic INSR. Endocrinology 159(1):465–476 [DOI] [PubMed] [Google Scholar]
- 44.Zaat TR, Kostova EB, Korsen P, Showell MG, Mol F, van Wely M (2023) Obstetric and neonatal outcomes after natural versus artificial cycle frozen embryo transfer and the role of luteal phase support: a systematic review and meta-analysis. Hum Reprod Update 29(5):634–654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu H, Zheng J, Liao AH (2022) The regulation and potential roles of m6A modifications in early embryonic development and immune tolerance at the maternal-fetal interface. Front Immunol 13:988130 [DOI] [PMC free article] [PubMed]
- 46.Li XC, Jin F, Wang BY, Yin XJ, Hong W, Tian FJ (2019) The m6A demethylase ALKBH5 controls trophoblast invasion at the maternal-fetal interface by regulating the stability of CYR61 mRNA. Theranostics 9(13):3853–3865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sheng Y, Wei JB, Yu F, Xu HZ, Yu CJ, Wu Q, Liu Y, Li L, Cui XL, Gu XY et al (2021) A critical role of nuclear m(6)a reader YTHDC1 in leukemogenesis by regulating MCM complex-mediated DNA replication. Blood 138(26):2838–2852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dietrich B, Haider S, Meinhardt G, Pollheimer J, Knofler M (2022) WNT and NOTCH signaling in human trophoblast development and differentiation. Cell Mol Life Sci 79(6) [DOI] [PMC free article] [PubMed]
- 49.Liu M, Liao LY, Gao YJ, Yin YX, Wei XH, Xu Q, Gao LB, Zhou R (2022) BCAM Deficiency May contribute to Preeclampsia by suppressing the PIK3R6/p-STAT3 signaling. Hypertension 79(12):2830–2842 [DOI] [PubMed] [Google Scholar]
- 50.Dong SS, Wang RR, Wang H, Ding Q, Zhou X, Wang J, Zhang KQ, Long Y, Lu S, Hong T et al (2019) HOXD-AS1 promotes the epithelial to mesenchymal transition of ovarian cancer cells by regulating mir-186-5p and PIK3R3. J Exp Clin Canc Res 38:110 [DOI] [PMC free article] [PubMed]
- 51.Wang WT, Huang ZP, Sui S, Liu JH, Yu DM, Wang WB (2020) microRNA-1236 promotes chondrocyte apoptosis in osteoarthritis via direct suppression of PIK3R3. Life Sci 253:117694 [DOI] [PubMed]
- 52.Mouton AJ, Ma YG, Gonzalez OJR, Daseke MJ, Flynn ER, Freeman TC, Garrett MR, DeLeon-Pennell KY, Lindsey ML (2019) Fibroblast polarization over the myocardial infarction time continuum shifts roles from inflammation to angiogenesis. Basic Res Cardiol 114(2) [DOI] [PMC free article] [PubMed]
- 53.Biffo S, Manfrini N, Ricciardi S (2018) Crosstalks between translation and metabolism in cancer. Curr Opin Genet Dev 48:75–81 [DOI] [PubMed] [Google Scholar]
- 54.Chu J, Cargnello M, Topisirovic I, Pelletier J (2016) Translation initiation factors: reprogramming protein synthesis in Cancer. Trends Cell Biol 26(12):918–933 [DOI] [PubMed] [Google Scholar]
- 55.Ge XJ, Xue G, Ding Y, Li R, Hu KN, Xu TJ, Sun M, Liao W, Zhao B, Wen CY et al (2023) The loss of YTHDC1 in Gut macrophages exacerbates inflammatory Bowel Disease. Adv Sci 10(14) [DOI] [PMC free article] [PubMed]
- 56.Evans RM, Mangelsdorf DJ (2014) Nuclear receptors, RXR, and the Big Bang. Cell 157(1):255–266 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The sequencing data that support the findings of this study are listed in NCBI Gene Expression Omnibus (Accession no: GSE244402). Other data that support the findings of this study are available from the corresponding author upon reasonable request.