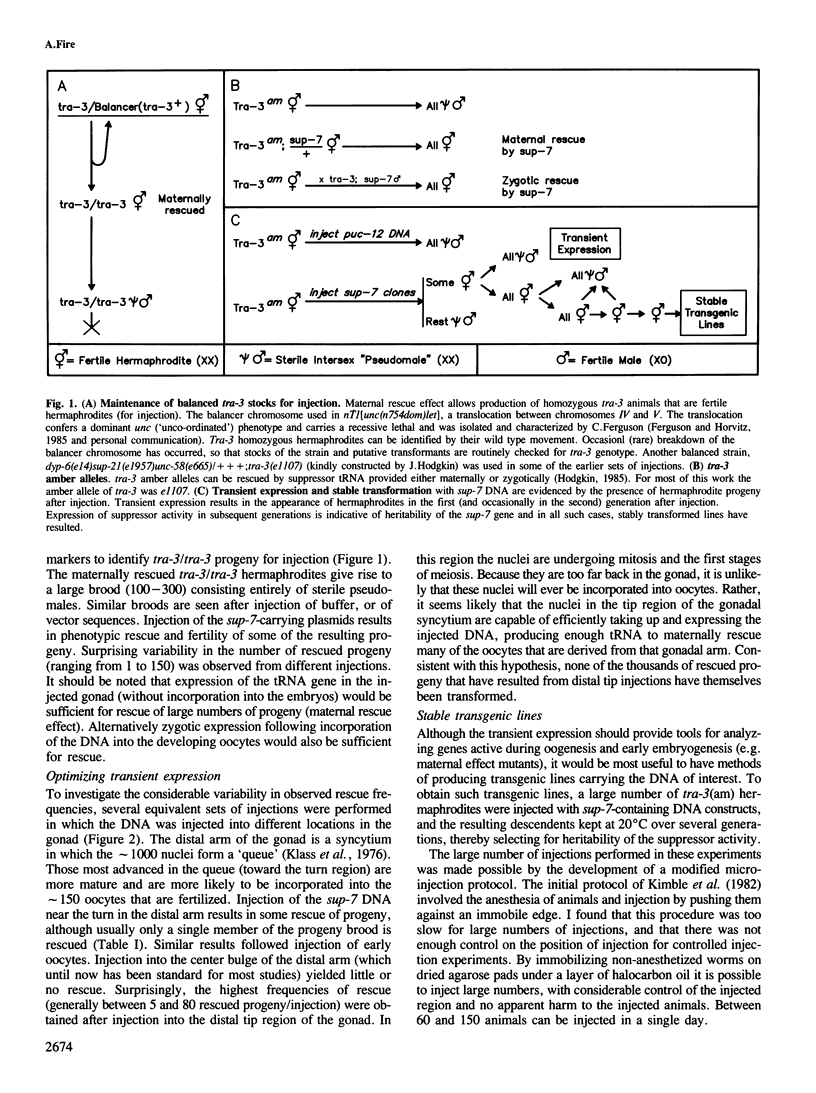
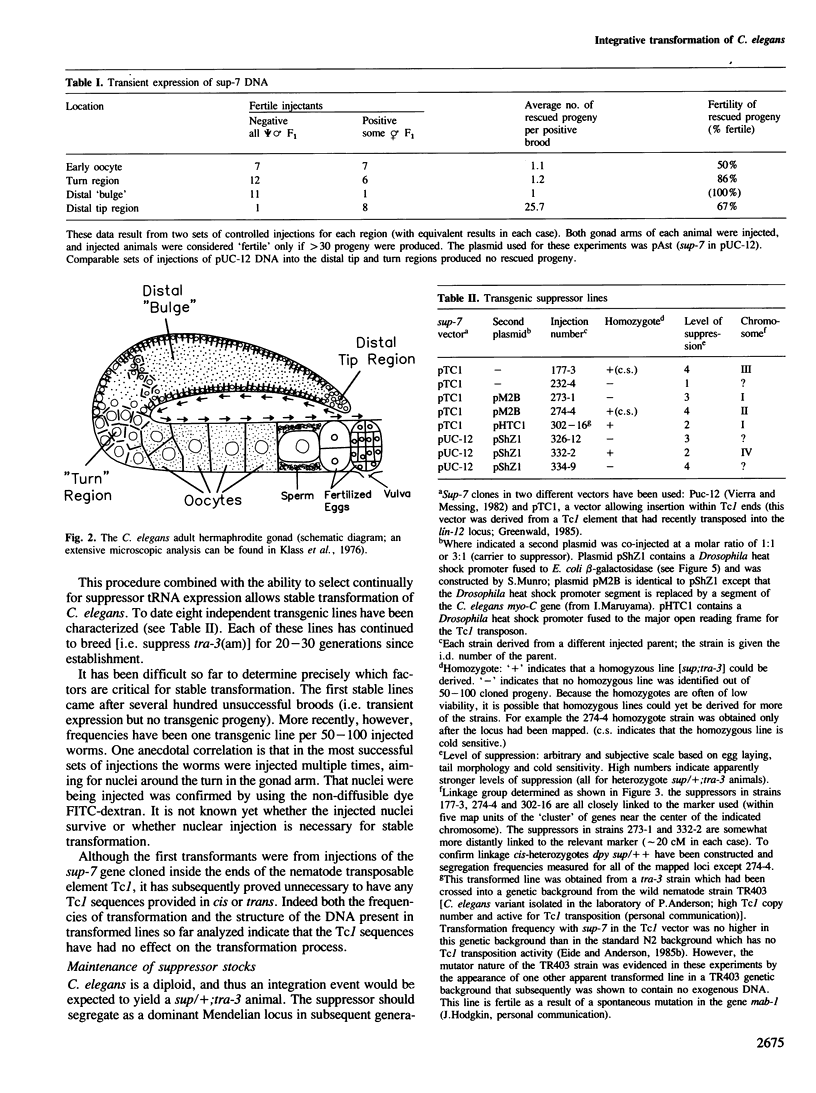
Abstract
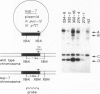
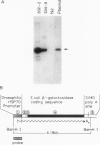
A technique for introducing exogenous DNA into the chromosomes of the nematode Caenorhabditis elegans is presented. A cloned C. elegans amber suppressor tRNA gene, sup-7, is used as a selectable marker. The activity of this amber suppressor is selected for by injecting worms which carry an amber termination mutation in a gene (tra-3) whose function is required for fertility. Transient expression of sup-7 is evidenced by the presence of fertile (rescued) animals in the generation after injection. In a fraction of cases, these fertile animals give rise to stable suppressor lines (eight have been characterized so far). Each of the stable suppressor lines carries injected DNA sequences. The suppressor activities have been mapped to chromosomal loci, indicating that the exogenous DNA has integrated into the genome. This technique has been used to introduce a chimeric gene containing a Drosophila heat shock promoter element fused to coding sequences from the Escherichia coli β-galactosidase gene. This chimeric gene functions and is heat inducible in the resulting stably transformed lines.
Keywords: C. elegans, transformation, amber suppressor, selection, integration
Full text
PDF

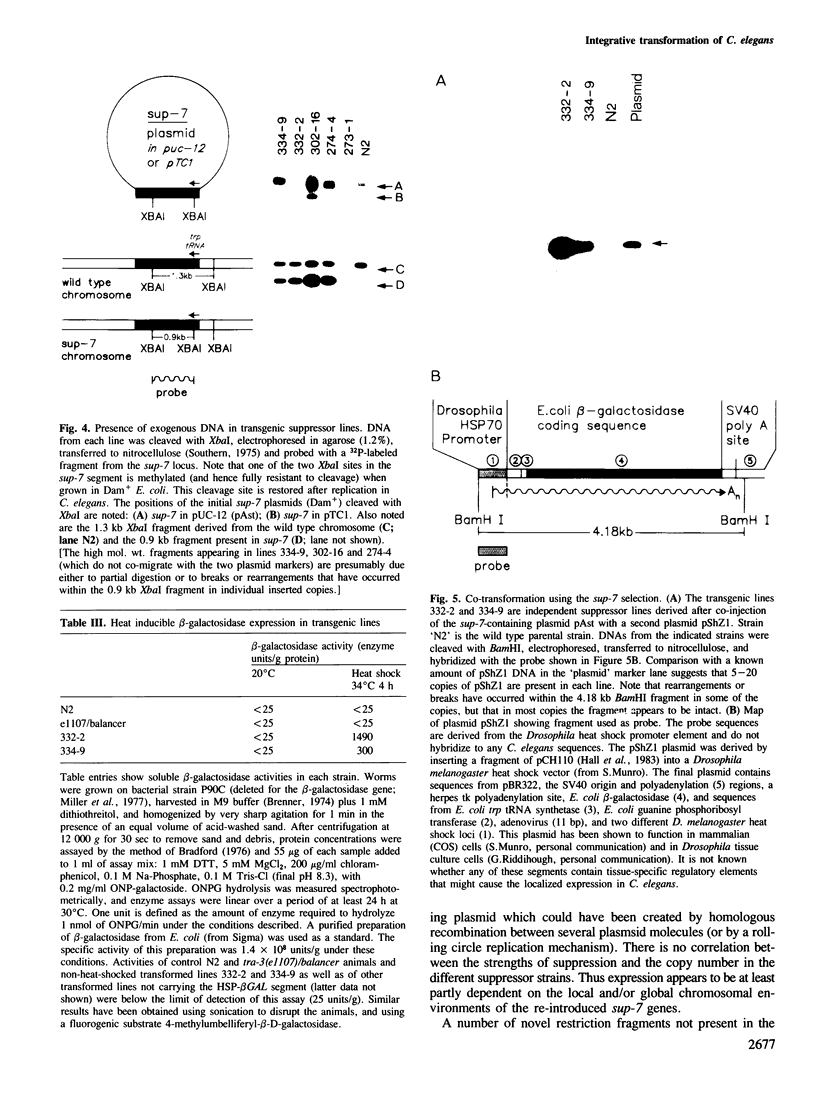



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birnboim H. C. A rapid alkaline extraction method for the isolation of plasmid DNA. Methods Enzymol. 1983;100:243–255. doi: 10.1016/0076-6879(83)00059-2. [DOI] [PubMed] [Google Scholar]
- Blumenthal T., Squire M., Kirtland S., Cane J., Donegan M., Spieth J., Sharrock W. Cloning of a yolk protein gene family from Caenorhabditis elegans. J Mol Biol. 1984 Mar 25;174(1):1–18. doi: 10.1016/0022-2836(84)90361-9. [DOI] [PubMed] [Google Scholar]
- Bolten S. L., Powell-Abel P., Fischhoff D. A., Waterston R. H. The sup-7(st5) X gene of Caenorhabditis elegans encodes a tRNATrpUAG amber suppressor. Proc Natl Acad Sci U S A. 1984 Nov;81(21):6784–6788. doi: 10.1073/pnas.81.21.6784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974 May;77(1):71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eide D., Anderson P. The gene structures of spontaneous mutations affecting a Caenorhabditis elegans myosin heavy chain gene. Genetics. 1985 Jan;109(1):67–79. doi: 10.1093/genetics/109.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eide D., Anderson P. Transposition of Tc1 in the nematode Caenorhabditis elegans. Proc Natl Acad Sci U S A. 1985 Mar;82(6):1756–1760. doi: 10.1073/pnas.82.6.1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmons S. W., Yesner L. High-frequency excision of transposable element Tc 1 in the nematode Caenorhabditis elegans is limited to somatic cells. Cell. 1984 Mar;36(3):599–605. doi: 10.1016/0092-8674(84)90339-8. [DOI] [PubMed] [Google Scholar]
- Emmons S. W., Yesner L., Ruan K. S., Katzenberg D. Evidence for a transposon in Caenorhabditis elegans. Cell. 1983 Jan;32(1):55–65. doi: 10.1016/0092-8674(83)90496-8. [DOI] [PubMed] [Google Scholar]
- Ferguson E. L., Horvitz H. R. Identification and characterization of 22 genes that affect the vulval cell lineages of the nematode Caenorhabditis elegans. Genetics. 1985 May;110(1):17–72. doi: 10.1093/genetics/110.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Files J. G., Carr S., Hirsh D. Actin gene family of Caenorhabditis elegans. J Mol Biol. 1983 Mar 5;164(3):355–375. doi: 10.1016/0022-2836(83)90056-6. [DOI] [PubMed] [Google Scholar]
- Greenwald I. S., Horvitz H. R. unc-93(e1500): A behavioral mutant of Caenorhabditis elegans that defines a gene with a wild-type null phenotype. Genetics. 1980 Sep;96(1):147–164. doi: 10.1093/genetics/96.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwald I. lin-12, a nematode homeotic gene, is homologous to a set of mammalian proteins that includes epidermal growth factor. Cell. 1985 Dec;43(3 Pt 2):583–590. doi: 10.1016/0092-8674(85)90230-2. [DOI] [PubMed] [Google Scholar]
- Hall C. V., Jacob P. E., Ringold G. M., Lee F. Expression and regulation of Escherichia coli lacZ gene fusions in mammalian cells. J Mol Appl Genet. 1983;2(1):101–109. [PubMed] [Google Scholar]
- Hirsh D., Kemphues K. J., Stinchcomb D. T., Jefferson R. Genes affecting early development in Caenorhabditis elegans. Cold Spring Harb Symp Quant Biol. 1985;50:69–78. doi: 10.1101/sqb.1985.050.01.011. [DOI] [PubMed] [Google Scholar]
- Hodgkin J. A., Brenner S. Mutations causing transformation of sexual phenotype in the nematode Caenorhabditis elegans. Genetics. 1977 Jun;86(2 Pt 1):275–287. [PMC free article] [PubMed] [Google Scholar]
- Hodgkin J. Male Phenotypes and Mating Efficiency in CAENORHABDITIS ELEGANS. Genetics. 1983 Jan;103(1):43–64. doi: 10.1093/genetics/103.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgkin J. Novel nematode amber suppressors. Genetics. 1985 Oct;111(2):287–310. doi: 10.1093/genetics/111.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgkin J. Two types of sex determination in a nematode. Nature. 1983 Jul 21;304(5923):267–268. doi: 10.1038/304267a0. [DOI] [PubMed] [Google Scholar]
- Karn J., Brenner S., Barnett L. Protein structural domains in the Caenorhabditis elegans unc-54 myosin heavy chain gene are not separated by introns. Proc Natl Acad Sci U S A. 1983 Jul;80(14):4253–4257. doi: 10.1073/pnas.80.14.4253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemphues K. J., Wolf N., Wood W. B., Hirsh D. Two loci required for cytoplasmic organization in early embryos of Caenorhabditis elegans. Dev Biol. 1986 Feb;113(2):449–460. doi: 10.1016/0012-1606(86)90180-6. [DOI] [PubMed] [Google Scholar]
- Kimble J., Hodgkin J., Smith T., Smith J. Suppression of an amber mutation by microinjection of suppressor tRNA in C. elegans. Nature. 1982 Sep 30;299(5882):456–458. doi: 10.1038/299456a0. [DOI] [PubMed] [Google Scholar]
- Klass M., Wolf N., Hirsh D. Development of the male reproductive system and sexual transformation in the nematode Caenorhabditis elegans. Dev Biol. 1976 Aug;52(1):1–18. doi: 10.1016/0012-1606(76)90002-6. [DOI] [PubMed] [Google Scholar]
- Kramer J. M., Cox G. N., Hirsh D. Comparisons of the complete sequences of two collagen genes from Caenorhabditis elegans. Cell. 1982 Sep;30(2):599–606. doi: 10.1016/0092-8674(82)90256-2. [DOI] [PubMed] [Google Scholar]
- Levis R., Hazelrigg T., Rubin G. M. Effects of genomic position on the expression of transduced copies of the white gene of Drosophila. Science. 1985 Aug 9;229(4713):558–561. doi: 10.1126/science.2992080. [DOI] [PubMed] [Google Scholar]
- Miller J. H., Ganem D., Lu P., Schmitz A. Genetic studies of the lac repressor. I. Correlation of mutational sites with specific amino acid residues: construction of a colinear gene-protein map. J Mol Biol. 1977 Jan 15;109(2):275–298. doi: 10.1016/s0022-2836(77)80034-x. [DOI] [PubMed] [Google Scholar]
- Moerman D. G., Benian G. M., Waterston R. H. Molecular cloning of the muscle gene unc-22 in Caenorhabditis elegans by Tc1 transposon tagging. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2579–2583. doi: 10.1073/pnas.83.8.2579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RUTENBURG A. M., RUTENBURG S. H., MONIS B., TEAGUE R., SELIGMAN A. M. Histochemical demonstration of beta-D-galactosidase in the rat. J Histochem Cytochem. 1958 Mar;6(2):122–129. doi: 10.1177/6.2.122. [DOI] [PubMed] [Google Scholar]
- Rubin G. M., Spradling A. C. Vectors for P element-mediated gene transfer in Drosophila. Nucleic Acids Res. 1983 Sep 24;11(18):6341–6351. doi: 10.1093/nar/11.18.6341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snutch T. P., Baillie D. L. Alterations in the pattern of gene expression following heat shock in the nematode Caenorhabditis elegans. Can J Biochem Cell Biol. 1983 Jun;61(6):480–487. doi: 10.1139/o83-064. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Stewart W. W. Functional connections between cells as revealed by dye-coupling with a highly fluorescent naphthalimide tracer. Cell. 1978 Jul;14(3):741–759. doi: 10.1016/0092-8674(78)90256-8. [DOI] [PubMed] [Google Scholar]
- Stinchcomb D. T., Shaw J. E., Carr S. H., Hirsh D. Extrachromosomal DNA transformation of Caenorhabditis elegans. Mol Cell Biol. 1985 Dec;5(12):3484–3496. doi: 10.1128/mcb.5.12.3484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira J., Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982 Oct;19(3):259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- Waterston R. H. A second informational suppressor, SUP-7 X, in Caenorhabditis elegans. Genetics. 1981 Feb;97(2):307–325. doi: 10.1093/genetics/97.2.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigler M., Sweet R., Sim G. K., Wold B., Pellicer A., Lacy E., Maniatis T., Silverstein S., Axel R. Transformation of mammalian cells with genes from procaryotes and eucaryotes. Cell. 1979 Apr;16(4):777–785. doi: 10.1016/0092-8674(79)90093-x. [DOI] [PubMed] [Google Scholar]
- Wills N., Gesteland R. F., Karn J., Barnett L., Bolten S., Waterston R. H. The genes sup-7 X and sup-5 III of C. elegans suppress amber nonsense mutations via altered transfer RNA. Cell. 1983 Jun;33(2):575–583. doi: 10.1016/0092-8674(83)90438-5. [DOI] [PubMed] [Google Scholar]