Abstract
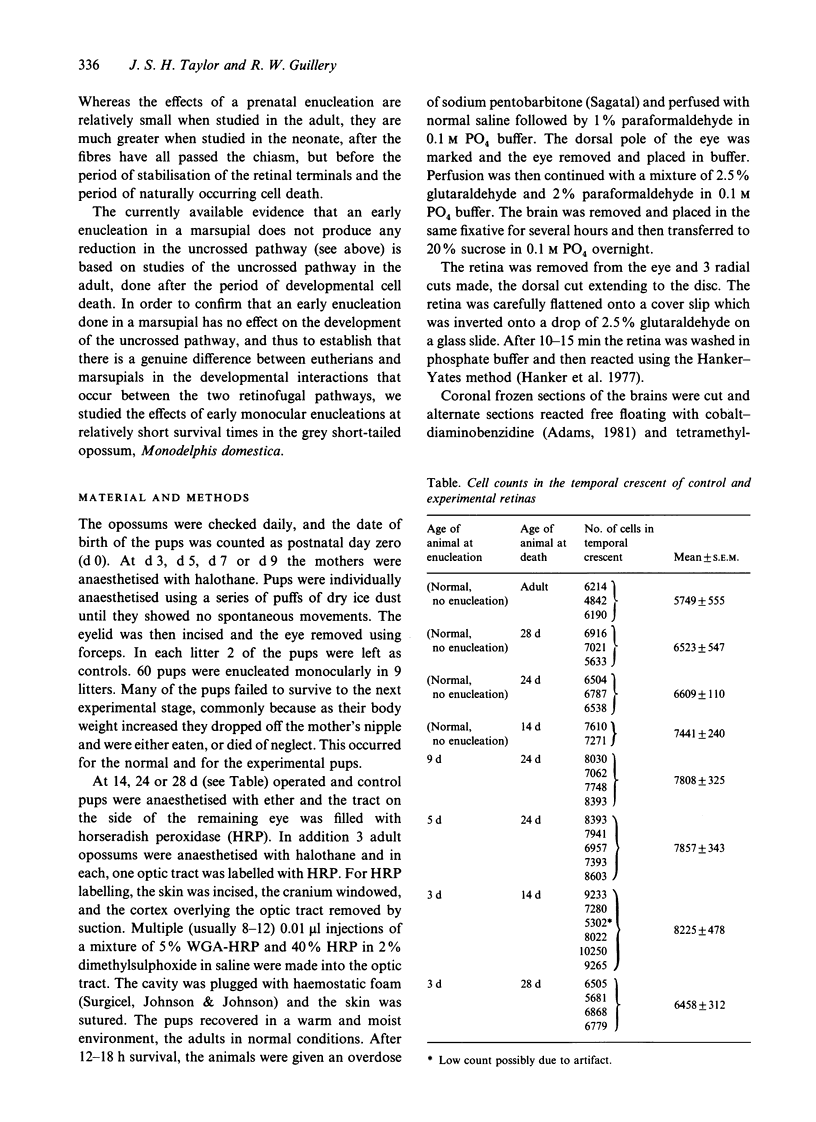
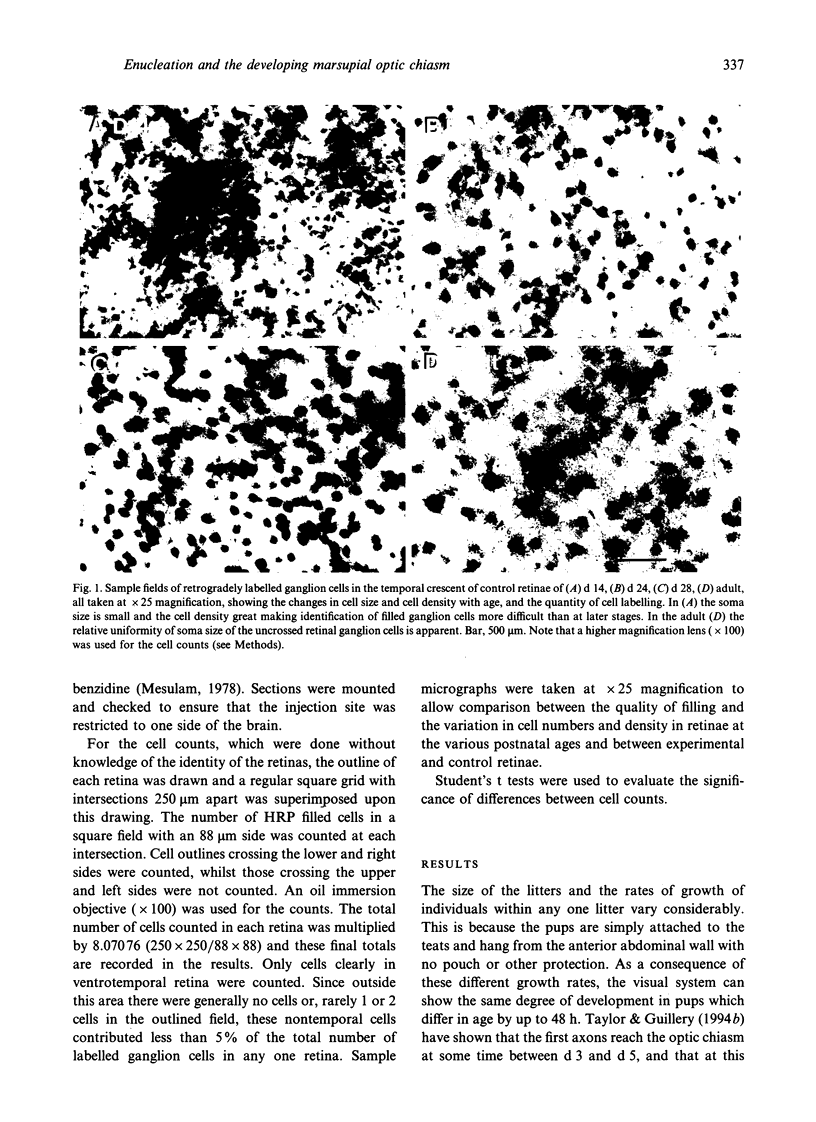
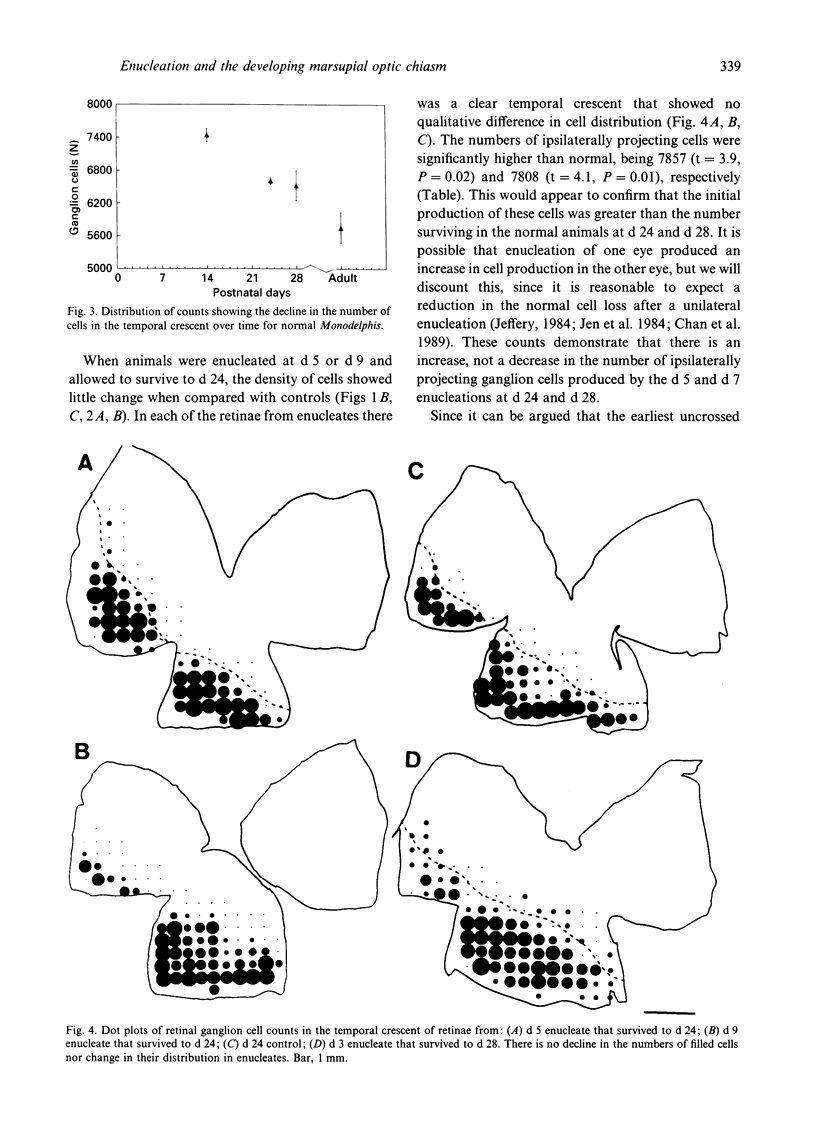
Monocular enucleations have been done during early stages (postnatal days 3 to 9) of visual system development of Monodelphis domestica, in order to determine whether in this marsupial, as in several eutherian mammals, there are any interactions between the pathways from the two eyes in establishing the uncrossed retinofugal projection. We have examined the distribution and the number of retrogradely labelled ganglion cells that project to the same side of the brain from the surviving eyes shortly after the uncrossed pathway is first formed in normal development (postnatal days 14 to 28). Even at these early stages of development the surviving uncrossed pathway shows no significant reduction, confirming earlier observations of adult marsupials and showing that at no stage in development is there any evidence that the crossed pathway from one eye influences the navigation of axons that will form the uncrossed pathway from the other eye. This is in sharp contrast to observations of mice, rats and ferrets and is in accord with expectations based on the difference of the chiasmatic structure in marsupials as compared with eutherians.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams J. C. Heavy metal intensification of DAB-based HRP reaction product. J Histochem Cytochem. 1981 Jun;29(6):775–775. doi: 10.1177/29.6.7252134. [DOI] [PubMed] [Google Scholar]
- Baker G. E., Reese B. E. Chiasmatic course of temporal retinal axons in the developing ferret. J Comp Neurol. 1993 Apr 1;330(1):95–104. doi: 10.1002/cne.903300108. [DOI] [PubMed] [Google Scholar]
- Chan S. O., Chow K. L., Jen L. S. Postnatal development of the ipsilaterally projecting retinal ganglion cells in normal rats and rats with neonatal lesions. Brain Res Dev Brain Res. 1989 Oct 1;49(2):265–274. doi: 10.1016/0165-3806(89)90027-8. [DOI] [PubMed] [Google Scholar]
- Chan S. O., Guillery R. W. Changes in fiber order in the optic nerve and tract of rat embryos. J Comp Neurol. 1994 Jun 1;344(1):20–32. doi: 10.1002/cne.903440103. [DOI] [PubMed] [Google Scholar]
- Chan S. O., Guillery R. W. Developmental changes produced in the retinofugal pathways of rats and ferrets by early monocular enucleations: the effects of age and the differences between normal and albino animals. J Neurosci. 1993 Dec;13(12):5277–5293. doi: 10.1523/JNEUROSCI.13-12-05277.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colello R. J., Guillery R. W. The early development of retinal ganglion cells with uncrossed axons in the mouse: retinal position and axonal course. Development. 1990 Mar;108(3):515–523. doi: 10.1242/dev.108.3.515. [DOI] [PubMed] [Google Scholar]
- Coleman L. A., Beazley L. D. Retinal ganglion cell number is unchanged in the remaining eye following early unilateral eye removal in the wallaby Setonix brachyurus, quokka. Brain Res Dev Brain Res. 1989 Aug 1;48(2):293–307. doi: 10.1016/0165-3806(89)90083-7. [DOI] [PubMed] [Google Scholar]
- Cucchiaro J., Guillery R. W. The development of the retinogeniculate pathways in normal and albino ferrets. Proc R Soc Lond B Biol Sci. 1984 Dec 22;223(1231):141–164. doi: 10.1098/rspb.1984.0087. [DOI] [PubMed] [Google Scholar]
- Godement P., Salaün J., Mason C. A. Retinal axon pathfinding in the optic chiasm: divergence of crossed and uncrossed fibers. Neuron. 1990 Aug;5(2):173–186. doi: 10.1016/0896-6273(90)90307-2. [DOI] [PubMed] [Google Scholar]
- Godement P., Salaün J., Métin C. Fate of uncrossed retinal projections following early or late prenatal monocular enucleation in the mouse. J Comp Neurol. 1987 Jan 1;255(1):97–109. doi: 10.1002/cne.902550108. [DOI] [PubMed] [Google Scholar]
- Godement P., Wang L. C., Mason C. A. Retinal axon divergence in the optic chiasm: dynamics of growth cone behavior at the midline. J Neurosci. 1994 Nov;14(11 Pt 2):7024–7039. doi: 10.1523/JNEUROSCI.14-11-07024.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillery R. W. Early monocular enucleations in fetal ferrets produce a decrease of uncrossed and an increase of crossed retinofugal components: a possible model for the albino abnormality. J Anat. 1989 Jun;164:73–84. [PMC free article] [PubMed] [Google Scholar]
- Guillery R. W., Taylor J. S. Different rates of axonal degeneration in the crossed and uncrossed retinofugal pathways of Monodelphis domestica. J Neurocytol. 1993 Sep;22(9):707–716. doi: 10.1007/BF01181316. [DOI] [PubMed] [Google Scholar]
- Hanker J. S., Yates P. E., Metz C. B., Rustioni A. A new specific, sensitive and non-carcinogenic reagent for the demonstration of horseradish peroxidase. Histochem J. 1977 Nov;9(6):789–792. doi: 10.1007/BF01003075. [DOI] [PubMed] [Google Scholar]
- Jeffery G., Harman A. M. Distinctive pattern of organisation in the retinofugal pathway of a marsupial: II. Optic chiasm. J Comp Neurol. 1992 Nov 1;325(1):57–67. doi: 10.1002/cne.903250106. [DOI] [PubMed] [Google Scholar]
- Jeffery G. Retinal ganglion cell death and terminal field retraction in the developing rodent visual system. Brain Res. 1984 Mar;315(1):81–96. doi: 10.1016/0165-3806(84)90079-8. [DOI] [PubMed] [Google Scholar]
- Jen L. S., So K. F., Woo H. H. An anterograde HRP study of the retinocollicular pathways in normal hamsters and hamsters with one eye enucleated at birth. Brain Res. 1984 Feb 27;294(1):169–173. doi: 10.1016/0006-8993(84)91325-8. [DOI] [PubMed] [Google Scholar]
- Lund R. D., Lund J. S. Reorganization of the retinotectal pathway in rats after neonatal retinal lesions. Exp Neurol. 1973 Aug;40(2):377–390. doi: 10.1016/0014-4886(73)90081-2. [DOI] [PubMed] [Google Scholar]
- Mesulam M. M. The blue reaction product in horseradish peroxidase neurohistochemistry: incubation parameters and visibility. J Histochem Cytochem. 1976 Dec;24(12):1273–1280. doi: 10.1177/24.12.63512. [DOI] [PubMed] [Google Scholar]
- Méndez-Otero R., Rocha-Miranda C. E., Carvalho-Dias E. Effects of monocular enucleation at different stages of development on the uncrossed retinocollicular projection in the opossum. Brain Res. 1986 Jun;392(1-2):101–108. doi: 10.1016/0165-3806(86)90236-1. [DOI] [PubMed] [Google Scholar]
- Reese B. E., Maynard T. M., Hocking D. R. Glial domains and axonal reordering in the chiasmatic region of the developing ferret. J Comp Neurol. 1994 Nov 8;349(2):303–324. doi: 10.1002/cne.903490211. [DOI] [PubMed] [Google Scholar]
- Sretavan D. W., Reichardt L. F. Time-lapse video analysis of retinal ganglion cell axon pathfinding at the mammalian optic chiasm: growth cone guidance using intrinsic chiasm cues. Neuron. 1993 Apr;10(4):761–777. doi: 10.1016/0896-6273(93)90176-r. [DOI] [PubMed] [Google Scholar]
- Taylor J. S., Guillery R. W. Early development of the optic chiasm in the gray short-tailed opossum, Monodelphis domestica. J Comp Neurol. 1994 Dec 1;350(1):109–121. doi: 10.1002/cne.903500108. [DOI] [PubMed] [Google Scholar]
- Taylor J. S., Guillery R. W. Early development of the optic chiasm in the gray short-tailed opossum, Monodelphis domestica. J Comp Neurol. 1994 Dec 1;350(1):109–121. doi: 10.1002/cne.903500108. [DOI] [PubMed] [Google Scholar]
- Walsh C., Guillery R. W. Age-related fiber order in the optic tract of the ferret. J Neurosci. 1985 Nov;5(11):3061–3069. doi: 10.1523/JNEUROSCI.05-11-03061.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]




