Abstract
Histochemical methods were used to assess the distribution of 4 neurotransmitters thought to be involved in cortical plasticity. They were measured in the primary auditory cortex of the ferret from just before the onset of hearing. Acetylcholinesterase staining was strongest in layers I, IV and VI and there was a gradual increase in the amount of staining from postnatal day (PND) 21 through to adulthood. Serotonin fibres were located mainly in layers I-III and their density increased gradually over the same time period. Noradrenergic fibres were sparsely scattered throughout the cortex but their density and distribution showed little change over the age range studied. Dopaminergic fibres were densest in layers V and VI at all ages. However, there was a transient doubling in their density that started round about the onset of hearing at PND 28, peaked at PND 35 and had returned to baseline levels by 2 wk later. This transient peak in density did not occur in the adjacent suprasylvian gyrus and did not appear to be a general phenomenon. The local transient increase in dopaminergic fibres implies that they may have an important role during a short period in auditory cortical development. This role may involve modifying the cortical circuitry that is involved in analysing the input from the auditory periphery.
Full text
PDF


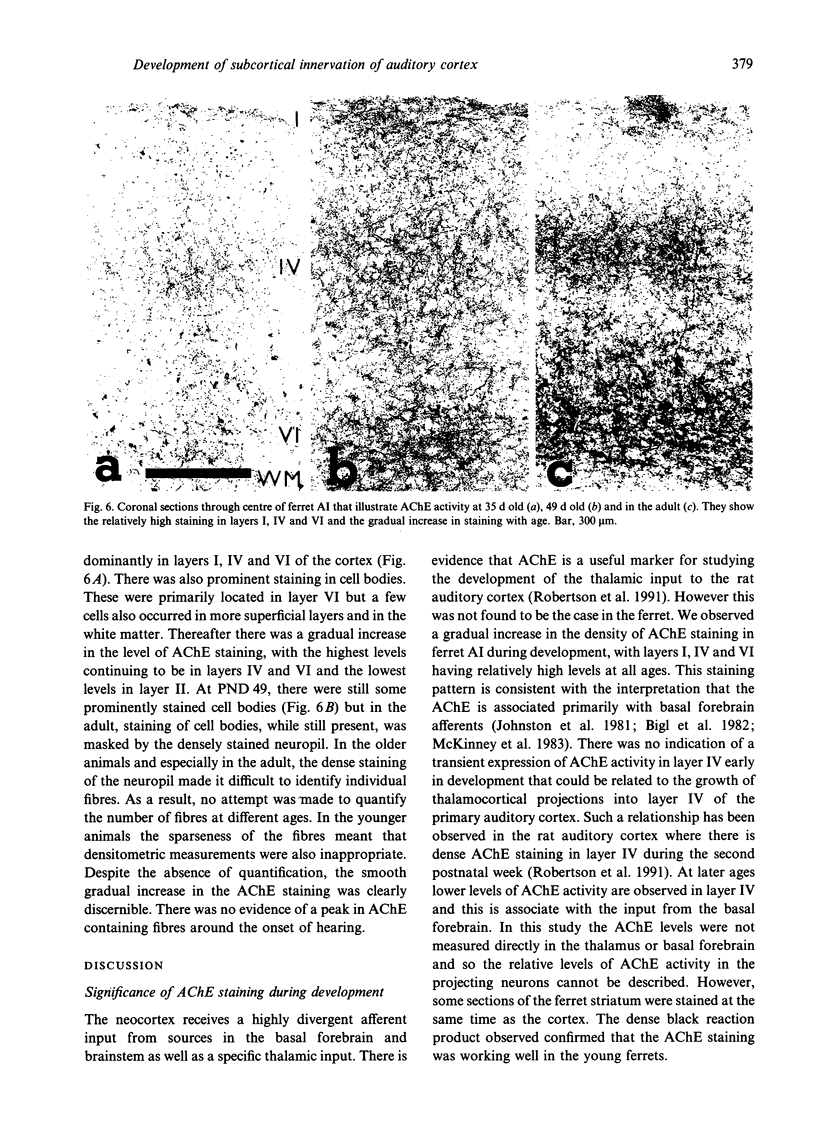



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bigl V., Woolf N. J., Butcher L. L. Cholinergic projections from the basal forebrain to frontal, parietal, temporal, occipital, and cingulate cortices: a combined fluorescent tracer and acetylcholinesterase analysis. Brain Res Bull. 1982 Jun;8(6):727–749. doi: 10.1016/0361-9230(82)90101-0. [DOI] [PubMed] [Google Scholar]
- Campbell M. J., Lewis D. A., Foote S. L., Morrison J. H. Distribution of choline acetyltransferase-, serotonin-, dopamine-beta-hydroxylase-, tyrosine hydroxylase-immunoreactive fibers in monkey primary auditory cortex. J Comp Neurol. 1987 Jul 8;261(2):209–220. doi: 10.1002/cne.902610204. [DOI] [PubMed] [Google Scholar]
- D'Amato R. J., Blue M. E., Largent B. L., Lynch D. R., Ledbetter D. J., Molliver M. E., Snyder S. H. Ontogeny of the serotonergic projection to rat neocortex: transient expression of a dense innervation to primary sensory areas. Proc Natl Acad Sci U S A. 1987 Jun;84(12):4322–4326. doi: 10.1073/pnas.84.12.4322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ericson H., Blomqvist A., Köhler C. Brainstem afferents to the tuberomammillary nucleus in the rat brain with special reference to monoaminergic innervation. J Comp Neurol. 1989 Mar 8;281(2):169–192. doi: 10.1002/cne.902810203. [DOI] [PubMed] [Google Scholar]
- Foote S. L., Bloom F. E., Aston-Jones G. Nucleus locus ceruleus: new evidence of anatomical and physiological specificity. Physiol Rev. 1983 Jul;63(3):844–914. doi: 10.1152/physrev.1983.63.3.844. [DOI] [PubMed] [Google Scholar]
- Foote S. L., Morrison J. H. Extrathalamic modulation of cortical function. Annu Rev Neurosci. 1987;10:67–95. doi: 10.1146/annurev.ne.10.030187.000435. [DOI] [PubMed] [Google Scholar]
- Foote S. L., Morrison J. H. Postnatal development of laminar innervation patterns by monoaminergic fibers in monkey (Macaca fascicularis) primary visual cortex. J Neurosci. 1984 Nov;4(11):2667–2680. doi: 10.1523/JNEUROSCI.04-11-02667.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaspar P., Berger B., Febvret A., Vigny A., Henry J. P. Catecholamine innervation of the human cerebral cortex as revealed by comparative immunohistochemistry of tyrosine hydroxylase and dopamine-beta-hydroxylase. J Comp Neurol. 1989 Jan 8;279(2):249–271. doi: 10.1002/cne.902790208. [DOI] [PubMed] [Google Scholar]
- Hubel D. H., Wiesel T. N. The period of susceptibility to the physiological effects of unilateral eye closure in kittens. J Physiol. 1970 Feb;206(2):419–436. doi: 10.1113/jphysiol.1970.sp009022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Innocenti G. M. Growth and reshaping of axons in the establishment of visual callosal connections. Science. 1981 May 15;212(4496):824–827. doi: 10.1126/science.7221566. [DOI] [PubMed] [Google Scholar]
- Jackson C. A., Peduzzi J. D., Hickey T. L. Visual cortex development in the ferret. I. Genesis and migration of visual cortical neurons. J Neurosci. 1989 Apr;9(4):1242–1253. doi: 10.1523/JNEUROSCI.09-04-01242.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston M. V., McKinney M., Coyle J. T. Neocortical cholinergic innervation: a description of extrinsic and intrinsic components in the rat. Exp Brain Res. 1981;43(2):159–172. doi: 10.1007/BF00237760. [DOI] [PubMed] [Google Scholar]
- Kalsbeek A., Matthijssen M. A., Uylings H. B. Morphometric analysis of prefrontal cortical development following neonatal lesioning of the dopaminergic mesocortical projection. Exp Brain Res. 1989;78(2):279–289. doi: 10.1007/BF00228899. [DOI] [PubMed] [Google Scholar]
- Kalsbeek A., Voorn P., Buijs R. M., Pool C. W., Uylings H. B. Development of the dopaminergic innervation in the prefrontal cortex of the rat. J Comp Neurol. 1988 Mar 1;269(1):58–72. doi: 10.1002/cne.902690105. [DOI] [PubMed] [Google Scholar]
- Kasamatsu T., Pettigrew J. D., Ary M. Restoration of visual cortical plasticity by local microperfusion of norepinephrine. J Comp Neurol. 1979 May 1;185(1):163–181. doi: 10.1002/cne.901850110. [DOI] [PubMed] [Google Scholar]
- Kelly J. B., Judge P. W., Phillips D. P. Representation of the cochlea in primary auditory cortex of the ferret (Mustela putorius). Hear Res. 1986;24(2):111–115. doi: 10.1016/0378-5955(86)90054-7. [DOI] [PubMed] [Google Scholar]
- Kossut M., Wójcik M., Skangiel-Kramska J. Dynamic changes of serotonin levels during the first visual experience of binocularly deprived kittens. J Neurochem. 1981 Nov;37(5):1077–1080. doi: 10.1111/j.1471-4159.1981.tb04656.x. [DOI] [PubMed] [Google Scholar]
- Lewis D. A., Campbell M. J., Foote S. L., Goldstein M., Morrison J. H. The distribution of tyrosine hydroxylase-immunoreactive fibers in primate neocortex is widespread but regionally specific. J Neurosci. 1987 Jan;7(1):279–290. doi: 10.1523/JNEUROSCI.07-01-00279.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luskin M. B., Shatz C. J. Neurogenesis of the cat's primary visual cortex. J Comp Neurol. 1985 Dec 22;242(4):611–631. doi: 10.1002/cne.902420409. [DOI] [PubMed] [Google Scholar]
- McKinney M., Coyle J. T., Hedreen J. C. Topographic analysis of the innervation of the rat neocortex and hippocampus by the basal forebrain cholinergic system. J Comp Neurol. 1983 Jun 10;217(1):103–121. doi: 10.1002/cne.902170109. [DOI] [PubMed] [Google Scholar]
- Moore D. R. Late onset of hearing in the ferret. Brain Res. 1982 Dec 16;253(1-2):309–311. doi: 10.1016/0006-8993(82)90698-9. [DOI] [PubMed] [Google Scholar]
- Morey A. L., Carlile S. Auditory brainstem of the ferret: maturation of the brainstem auditory evoked response. Brain Res Dev Brain Res. 1990 Mar 1;52(1-2):279–288. doi: 10.1016/0165-3806(90)90246-u. [DOI] [PubMed] [Google Scholar]
- Mower G. D. The effect of dark rearing on the time course of the critical period in cat visual cortex. Brain Res Dev Brain Res. 1991 Feb 22;58(2):151–158. doi: 10.1016/0165-3806(91)90001-y. [DOI] [PubMed] [Google Scholar]
- O'Hearn E., Molliver M. E. Organization of raphe-cortical projections in rat: a quantitative retrograde study. Brain Res Bull. 1984 Dec;13(6):709–726. doi: 10.1016/0361-9230(84)90232-6. [DOI] [PubMed] [Google Scholar]
- Pallas S. L., Roe A. W., Sur M. Visual projections induced into the auditory pathway of ferrets. I. Novel inputs to primary auditory cortex (AI) from the LP/pulvinar complex and the topography of the MGN-AI projection. J Comp Neurol. 1990 Aug 1;298(1):50–68. doi: 10.1002/cne.902980105. [DOI] [PubMed] [Google Scholar]
- Phillips D. P., Judge P. W., Kelly J. B. Primary auditory cortex in the ferret (Mustela putorius): neural response properties and topographic organization. Brain Res. 1988 Mar 8;443(1-2):281–294. doi: 10.1016/0006-8993(88)91622-8. [DOI] [PubMed] [Google Scholar]
- Porrino L. J., Goldman-Rakic P. S. Brainstem innervation of prefrontal and anterior cingulate cortex in the rhesus monkey revealed by retrograde transport of HRP. J Comp Neurol. 1982 Feb 10;205(1):63–76. doi: 10.1002/cne.902050107. [DOI] [PubMed] [Google Scholar]
- ROSE J. E. The cellular structure of the auditory region of the cat. J Comp Neurol. 1949 Dec;91(3):409-39, incl 5 pl. doi: 10.1002/cne.900910305. [DOI] [PubMed] [Google Scholar]
- Reale R. A., Imig T. J. Tonotopic organization in auditory cortex of the cat. J Comp Neurol. 1980 Jul 15;192(2):265–291. doi: 10.1002/cne.901920207. [DOI] [PubMed] [Google Scholar]
- Robertson R. T., Mostamand F., Kageyama G. H., Gallardo K. A., Yu J. Primary auditory cortex in the rat: transient expression of acetylcholinesterase activity in developing geniculocortical projections. Brain Res Dev Brain Res. 1991 Jan 15;58(1):81–95. doi: 10.1016/0165-3806(91)90240-j. [DOI] [PubMed] [Google Scholar]
- Robertson R. T., Tijerina A. A., Gallivan M. E. Transient patterns of acetylcholinesterase activity in visual cortex of the rat: normal development and the effects of neonatal monocular enucleation. Brain Res. 1985 Aug;353(2):203–214. doi: 10.1016/0165-3806(85)90209-3. [DOI] [PubMed] [Google Scholar]
- Scheich H. Auditory cortex: comparative aspects of maps and plasticity. Curr Opin Neurobiol. 1991 Aug;1(2):236–247. doi: 10.1016/0959-4388(91)90084-k. [DOI] [PubMed] [Google Scholar]
- Schmidt R. H., Bhatnagar R. K. Assessment of the effects of neonatal subcutaneous 6-hydroxydopamine on noradrenergic and dopaminergic innervation of the cerebral cortex. Brain Res. 1979 Apr 27;166(2):309–319. doi: 10.1016/0006-8993(79)90216-6. [DOI] [PubMed] [Google Scholar]
- Schreiner C. E. Functional organization of the auditory cortex: maps and mechanisms. Curr Opin Neurobiol. 1992 Aug;2(4):516–521. doi: 10.1016/0959-4388(92)90190-v. [DOI] [PubMed] [Google Scholar]
- Voigt T., de Lima A. D. Serotoninergic innervation of the ferret cerebral cortex. II. Postnatal development. J Comp Neurol. 1991 Dec 8;314(2):415–428. doi: 10.1002/cne.903140215. [DOI] [PubMed] [Google Scholar]
- Vu D. H., Törk I. Differential development of the dual serotoninergic fiber system in the cerebral cortex of the cat. J Comp Neurol. 1992 Mar 8;317(2):156–174. doi: 10.1002/cne.903170205. [DOI] [PubMed] [Google Scholar]
- Wallace M. N., Bajwa S. Patchy intrinsic connections of the ferret primary auditory cortex. Neuroreport. 1991 Aug;2(8):417–420. doi: 10.1097/00001756-199108000-00001. [DOI] [PubMed] [Google Scholar]
- Wallace M. N., Kitzes L. M., Jones E. G. Chemoarchitectonic organization of the cat primary auditory cortex. Exp Brain Res. 1991;86(3):518–526. doi: 10.1007/BF00230525. [DOI] [PubMed] [Google Scholar]
- Wallace M. N., Kitzes L. M., Jones E. G. Intrinsic inter- and intralaminar connections and their relationship to the tonotopic map in cat primary auditory cortex. Exp Brain Res. 1991;86(3):527–544. doi: 10.1007/BF00230526. [DOI] [PubMed] [Google Scholar]