Abstract
Objective
To evaluate the benefits of surgical repair acute type A aortic dissection (ATAAD) on survival of octogenarians.
Methods
Patients who underwent surgery for acute ATAAD from the multicenter European Registry of Type A Aortic Dissection (ERTAAD) were the subjects of the present analysis.
Results
326 (8.4%) patients were aged ≥ 80 years. Among 280 propensity score matched pairs, in-hospital mortality was 30.0% in patients aged ≥ 80 years and 20.0% in younger patients (P = 0.006), while 10-year mortality were 93.2% and 48.0%, respectively (P < 0.001). The hazard of mortality was higher among octogenarians up to two years after surgery, but it became comparable to that of younger patients up to 5 years. Among patients who survived 3 months after surgery, 10-year relative survival was 0.77 in patients aged < 80 years, and 0.46 in patients aged ≥ 80 years. Relative survival of octogenarians decreased markedly 5 years after surgery. Age ≥ 85 years, glomerular filtration rate, preoperative invasive ventilation, preoperative mesenteric mal-perfusion and aortic root replacement were independent predictors of in-hospital mortality among octogenarians (AUC = 0.792; E:O ratio = 0.991; CITL = 0.016; slope = 1.096). An additive score was developed. A risk score ≤ 1 was observed in 68.4% of patients, and their in-hospital mortality was 20.9%.
Conclusions
Provided a thoughtful patient selection, surgery may provide a survival benefit in patients aged ≥ 80 years with ATAAD that, when compared to younger patients and the general population, may last up to 5 years after the procedure. These findings have significant epidemiologic and clinical relevance because of the increasing longevity of the population of the Western countries.
Healthy lifestyle and pharmaceutical innovation led to increasing longevity,[1–4] which inevitably resulted in an increasing need of invasive cardiovascular interventions.[5–7] The incidence of acute aortic syndromes is expected to increase in the elderly as well. Acute type A aortic dissection (ATAAD) requires emergency surgical repair with significant mortality, particularly among aged patients.[8] The decision-making process in the emergency setting for the treatment of ATAAD in octogenarians is based on limited clinical data,[9] controversial results[10-12] and debated ethical issues.[13] Prior studies focused on the evaluation of the early outcomes because the high incidence of early postoperative adverse events remains the main concern in these patients. Indeed, excessive rates of early mortality and major complications may question the value of major aortic surgery in frailty patients with limited life-expectancy. Furthermore, there are no data on the potential benefit of surgery for ATAAD in terms of late survival in these patients compared to younger patients and the matched general population, which may support clinical decisions. These issues have been investigated in the present study.
METHODS
Study Population
The European Registry of Type A Aortic Dissection (ERTAAD) is a retrospective, multicenter study including consecutive patients who underwent surgery for ATAAD at 18 centers of cardiac surgery in eight European countries from January 2005 to March 2021.[14] The ERTAAD was registered in ClinicalTrials.gov with the identifier NCT04831073. The Ethical Review Board of the Helsinki University Hospital, Finland (April 21, 2021, diary no. HUS/237/2021) and the Ethical Review Board of each participating hospital approved this study. The requirement for informed consent was waived because of the retrospective nature of this registry.
Inclusion and Exclusion Criteria
The inclusion criteria of the ERTAAD were the following: (1) patients who underwent surgery for ATAAD; (2) patients aged > 18 years old; (3) symptoms arisen within 7 days prior to surgery; (4) primary repair of ATAAD; (5) any other cardiac surgical procedure concomitant with surgery for ATAAD. The exclusion criteria of this registry were the following: (1) patients aged < 18 years; (2) symptoms arisen more than seven days prior to surgery; (3) previous procedure for ATAAD; (4) retrograde ATAAD; (5) concomitant endocarditis; and (6) ATAAD secondary to blunt or penetrating chest trauma.
Data on late mortality was gathered from electronic national registries, records of control visits as well as by contacting regional hospitals and patients or their relatives. The definition criteria for risk factors and outcomes of interest have been previously reported.[14] Estimated glomerular filtration rate was herein estimated according to the CKD-EPI equation.[15]
Surgical Procedures
Regarding the extent of surgical repair, partial aortic arch replacement referred to any procedure with distal anastomosis performed at the level of the Ishimaru zones 1 to 2, with reimplantation of at least one epiaortic vessel to the aortic graft. Total aortic arch replacement referred to procedures with a distal anastomosis at the level of the Ishimaru zones 3 to 4 and the frozen elephant trunk operation. Aortic root replacement was defined as any procedure involving the resection of the aortic root.
Study Outcomes
The primary outcomes of this study were in-hospital mortality, i.e., all-cause mortality during the index hospitalization, and 10-year all-cause mortality. Five-year mortality was the primary outcome in analyses of subset of patients of limited size. Secondary outcomes were stroke/global brain ischemia, paraplegia/paraparesis, tetraplegia/tetraparesis, mesenteric ischemia, sepsis, dialysis, acute reoperation for intrathoracic bleeding, tracheostomy, deep sternal wound infection, heart failure, need of mechanical circulatory support, upper and lower limb ischemia, major lower limb amputation and procedures for intestinal complications.
Statistical Analysis
Statistical analyses were performed with the SPSS (version 29.0, IBM SPSS statistics, Chicago, Illinois, USA), Stata (version 15.1, StataCorp LLC, College Station, Texas, USA) and Open Meta-analyst (http://www.cebm.brown.edu/openmeta/index.html; accessed January 13, 2024) statistical softwares. Categorical variables were reported as counts and percentages, while continuous variables were reported as means and standard deviations. The Chi-square test and the Fisher’s exact test were used to compare categorical variables, whilst the Mann-Whitney’s test was used to compare continuous variables. Differences in terms of in-hospital mortality between multi-categorical classes were assessed with the linear-by-linear association test. Multilevel mixed-effects logistic regression was performed to estimate the probability of being included in the study cohorts considering the cluster effects of participating hospitals. The following baseline and operative variables were included into the regression model considering a cutoff of 80 years of age as the dependent variable: preoperative eGFR, preoperative arterial lactate, genetic syndromes, bicuspid aortic valve, iatrogenic ATAAD, diabetes, extracardiac arteriopathy, prior stroke, pulmonary disease, use of inotropes, invasive mechanical ventilation, salvage procedure, cerebral malperfusion, renal malperfusion, mesenteric malperfusion and peripheral malperfusion, DeBakey type I aortic dissection, aortic root replacement, partial/total aortic arch replacement, concomitant coronary artery bypass grafting and concomitant mitral and/or tricuspid valve surgery. The use of hypothermic circulatory arrest and, consequently, of antegrade cerebral perfusion were not included in the model because their use was less frequent among octogenarians as an attempt to avoid a period of brain ischemia in these high-risk patients (Table 1). Discrimination of the logistic regression model was assessed by estimating the area under (AUC) the receiver operating characteristics (ROC) curve and its calibration using the Hosmer-Lemeshow’s test. One-to-one propensity score matching was performed using a caliper width of 0.2 the standard deviation of the estimated logit (i.e., 0.2). A non-significant imbalance of the covariates between the study cohorts was considered when standardized difference was lower than 0.10. Ten-year mortality of the study cohorts was estimated using the Kaplan-Meier’s methos and difference between study groups were assessed with the log-rank test. Competing risk analysis was used to evaluate the risk and cumulative incidence of distal and proximal aortic reoperations with the Fine-Gray’s test. Cumulative observed survival, country-, year-, age- and sex-matched cumulative expected survival and cumulative relative survival were estimated using the Estéve’s method[16] with the strel2 module for Stata.[17] Relative survival is the ratio of the proportion of observed survivors in a cohort of patients to the proportion of expected survivors in a comparable set of disease-free subjects. A relative survival < 1.0 suggests that the observed survival of patients is lower than the expected survival of disease-free subjects. Mortality rates of the general population of each country of the participating hospitals were retrieved from the Human Mortality Database.[18] Multilevel mixed effects logistic regression was used to identify the risk factors for in-hospital mortality among octogenarians considering the possible cluster effect of the centers (Table 1). Calibration plot, the expected/observed ratio, calibration-in-the-large and slope for the estimated probabilities of this regression model in predicting in-hospital mortality were calculated using the pmcalplot module for Stata software.[19] An additive risk score for in-hospital mortality among octogenarians was developed by rounding the estimated ORs divided by two. Risk estimates were reported as odds ratio (OR) with their 95% confidence interval (CI). Finally, since the proportion and in-hospital mortality of octogenarians varied between the participating hospitals (Table 1), we adopted a meta-analytic approach to investigate center-level effect on the in-hospital mortality of patients age ≥ 80 years. Analyses were conducted using the random-effects methods and heterogeneity was quantified with the I2 test. I2 > 40% was considered a significant heterogeneity between centers.
Table 1. Proportion of octogenarians and their in-hospital mortality rate at the participating hospitals.
| Hospitals | No. of ATAAD patients of any age | Octogenarians | In-hospital mortality |
| Data are presented as n (%). ATAAD: acute type A aortic dissection. | |||
| A | 132 | 18 (13.6%) | 8 (44.4%) |
| B | 156 | 4 (2.6%) | 1 (25.0%) |
| C | 249 | 26 (10.4%) | 7 (26.9%) |
| D | 69 | 2 (2.9%) | 1 (50.0%) |
| E | 281 | 18 (6.4%) | 3 (16.7%) |
| F | 329 | 15 (4.6%) | 7 (46.7%) |
| G | 133 | 11 (8.3%) | 6 (54.5%) |
| H | 172 | 4 (2.3%) | 1 (25.0%) |
| I | 105 | 6 (5.7%) | 2 (33.3%) |
| L | 341 | 29 (8.5%) | 6 (20.7%) |
| M | 308 | 15 (4.9%) | 6 (40.0%) |
| N | 167 | 22 (13.2%) | 9 (40.9%) |
| O | 293 | 29 (9.9%) | 16 (55.2%) |
| P | 81 | 7 (8.6%) | 5 (71.4%) |
| Q | 492 | 59 (12.0%) | 19 (32.2%) |
| R | 141 | 18 (12.8%) | 3 (16.7%) |
| S | 182 | 15 (8.2%) | 1 (6.7%) |
| T | 271 | 28 (10.3%) | 2 (7.1%) |
| Total | 3902 | 326 (8.4%) | 103 (31.6%) |
RESULTS
Patient Demographics
Overall, 3902 consecutive patients were included in the ERTAAD registry, and they fulfilled the inclusion criteria of the present analysis. The proportion of octogenarians was 8.4% (326/3902 patients) and significantly varied between participating hospitals from 2.3% to 13.6% (Linear-by-linear association test, P = 0.001) (Table 1). Three patients were nonagenarians.
Outcomes in the Overall Series
Among patients aged ≥ 80 years, crude in-hospital mortality rates varied from 6.7% to 71.4% between the participating hospitals (linear-by-linear association test P = 0.091) (Table 1). The pooled in-hospital mortality rate among octogenarians was 31.3% (95%CI: 23.0-40.2%, I2 = 61%). Univariable meta-regression showed that neither the annual volume of procedures in ATAAD patients of any age (P = 0.198) nor the annual volume of procedures in ATAAD patients aged ≥ 80 years (P = 0.368) were significantly associated with the risk of in-hospital mortality in these elderly.
The baseline characteristics and operative variables of patients aged ≥ 80 years and of those aged < 80 years are summarized in Table 2. In the overall series, crude in-hospital mortality was 31.6% in patients aged ≥ 80 years and 16.4% in younger patients (crude OR = 2.347, 95%CI: 1.829–3.013; P < 0.001). Five-year and 10-year mortality rates were 53.5% and 93.0% in patients aged ≥ 80 years, and 30.8% and 44.0% in patients aged < 80 years, respectively (P < 0.001).
Table 2. Patients’ characteristics and operative data of patients in the study cohorts.
| Unmatched cohorts | Propensity score matched cohorts | ||||||
| Age < 80 years n = 3576 |
Age≥ 80 years n = 326 |
Standardized differences |
Age < 80 years n = 280 |
Age≥ 80 years n = 280 |
Standardized differences |
||
| Data are presented as mean ± SD or n (%). eGFR: estimated glomerular filtration rate is according to the CKD-EPI equation. | |||||||
| Baseline characteristics | |||||||
| Age, yrs | 61.5 ± 12.1 | 83.2 ± 2.3 | 1.000 | 65.6 ± 10.6 | 83.3 ± 2.4 | 1.000 | |
| Females | 1016 (28.4%) | 169 (51.8%) | 0.492 | 144 (51.4%) | 135 (48.2%) | 0.064 | |
| eGFR, mL/min per 1.73 m2 | 70 ± 23 | 58 ± 18 | 0.574 | 61 ± 21 | 59 ± 18 | 0.089 | |
| Genetic syndrome | 81 (2.3%) | 0 | 0.215 | 0 | 0 | 0.000 | |
| Bicuspid aortic valve | 148 (3.9%) | 3 (0.9%) | 0.206 | 0 | 2 (0.7%) | 0.120 | |
| Iatrogenic dissection | 95 (2.7%) | 8 (2.5%) | 0.013 | 4 (1.4%) | 7 (2.5%) | 0.054 | |
| Diabetes | 174 (4.9%) | 22 (6.7%) | 0.080 | 23 (8.2%) | 17 (6.1%) | 0.083 | |
| Stroke | 136 (3.8%) | 17 (5.2%) | 0.070 | 10 (3.6%) | 14 (5.0%) | 0.071 | |
| Pulmonary disease | 295 (8.2%) | 32 (9.8%) | 0.055 | 27 (9.6%) | 29 (10.4%) | 0.024 | |
| Extracardiac arteriopathy | 173 (8.2%) | 26 (8.0%) | 0.128 | 23 (8.2%) | 20 (7.1%) | 0.040 | |
| Prior cardiac surgery | 113 (3.2%) | 9 (2.8%) | 0.024 | 4 (1.4%) | 6 (2.1%) | 0.054 | |
| Cardiac massage | 165 (4.6%) | 14 (4.3%) | 0.015 | 8 (2.9%) | 12 (4.3%) | 0.077 | |
| Shock requiring inotropes | 580 (16.2%) | 68 (20.9%) | 0.120 | 50 (17.9%) | 56 (20.0%) | 0.055 | |
| Invasive ventilatio | 314 (8.8%) | 41 (12.6%) | 0.123 | 47 (16.8%) | 37 (13.2%) | 0.100 | |
| Preoperative malperfusion | |||||||
| Cerebral | 761 (21.3%) | 69 (21.2%) | 0.003 | 68 (24.3%) | 62 (22.1%) | 0.051 | |
| Spinal | 79 (2.2%) | 3 (0.9%) | 0.104 | 1 (0.4%) | 3 (1.1%) | 0.085 | |
| Renal | 342 (9.6%) | 22 (6.7%) | 0.103 | 24 (8.6%) | 20 (7.1%) | 0.053 | |
| Mesenteric | 146 (4.1%) | 16 (4.9%) | 0.040 | 9 (3.2%) | 13 (4.6%) | 0.074 | |
| Peripheral | 516 (14.4%) | 28 (8.6%) | 0.184 | 24 (8.6%) | 26 (9.3%) | 0.025 | |
| DeBakey type I dissection | 3029 (84.7%) | 246 (75.5%) | 0.265 | 217 (78.1%) | 219 (78.2%) | 0.004 | |
| Operative data | |||||||
| Salvage procedure | 164 (4.6%) | 15 (4.6%) | 0.001 | 9 (3.2%) | 13 (4.6%) | 0.073 | |
| Partial/total arch replacement | 752 (21.0%) | 24 (7.4%) | 0.399 | 22 (7.9%) | 23 (8.2%) | 0.004 | |
| Aortic root replacement | 1056 (9.2%) | 24 (7.4%) | 0.066 | 37 (13.2%) | 39 (13.9%) | 0.021 | |
| Coronary surgery | 328 (9.2%) | 24 (7.4%) | 0.067 | 19 (6.8%) | 19 (6.8%) | 0.000 | |
| Mitral or tricuspid valve surgery | 28 (0.8%) | 5 (1.5%) | 0.070 | 1 (0.4%) | 2 (0.7%) | 0.049 | |
| Hypothermic circulatory arrest | 3150 (88.4%) | 255 (78.2%) | 0.274 | 242 (86.4%) | 218 (77.9%) | 0.225 | |
| Antegrade cerebral perfusion | 2382 (66.6%) | 199 (61.0%) | 0.116 | 189 (67.5%) | 170 (60.7%) | 0.141 | |
Propensity Score Matching Analysis
Comorbidities were more prevalent among octogenarians, while ATAAD-related critical clinical conditions were comparable to younger patients (Table 2). Partial or total aortic arch surgery was more frequently performed among patients aged < 80 years. Similarly, hypothermic circulatory arrest was more frequently used among younger patients along with higher proportion of patients who underwent surgery using antegrade cerebral perfusion. Because of differences in several baseline and operative covariates, a propensity score matching was performed, which yielded 280 pairs of patients with well-balanced characteristics distribution, except in terms of age and prevalence of bicuspid aortic valve (Table 2). In-hospital mortality was 20.0% in patients aged < 80 years and 30.0% in patients aged ≥ 80 years (adjusted OR = 1.714, 95% CI: 1.162–2.529, P = 0.006) (Table 2). Stroke was more frequent among younger patients (17.5% vs. 9.3%, P = 0.004), and patients aged ≥ 80 years had an increased risk of deep sternal wound infection (3.6% vs. 0.4%, P = 0.006). The incidence of other adverse events was comparable between the matched study groups (Table 3). Ten-year cumulative incidences of distal aortic reoperations in patients aged ≥ 80 years and younger patients were 0.5% and 6.0%, respectively (P = 0.015), while cumulative incidences of proximal aortic reoperation were 0% and 3.6%, respectively (P < 0.001).
Table 3. Outcomes of patients in the study cohorts.
| Outcomes | Unmatched cohorts | Propensity score matched cohorts | |||||
| Age < 80 years n = 3576 |
Age ≥ 80 years n = 326 |
P-value | Age < 80 years n = 280 |
Age ≥ 80 years n = 280 |
P-value | ||
| Data are presented as n (%). | |||||||
| Hospital mortality | 586 (16.4%) | 103 (31.6%) | < 0.001 | 56 (20.0%) | 84 (30.0%) | 0.006 | |
| Stroke/global brain ischemia | 676 (18.9%) | 47 (14.4%) | 0.046 | 59 (21.1%) | 35 (12.5%) | 0.007 | |
| Paraparesis/paraplegia | 195 (5.5%) | 9 (2.8%) | 0.037 | 12 (4.3%) | 8 (2.9%) | 0.362 | |
| Tetraparesis/tetraplegia | 3 (0.1%) | 0 | 1.000 | 0 | 0 | - | |
| Mesenteric ischemia | 131 (3.7%) | 18 (5.5%) | 0.094 | 12 (4.3%) | 15 (5.4%) | 0.554 | |
| Sepsis | 440 (12.3%) | 34 (10.4%) | 0.321 | 35 (12.5%) | 31 (11.1%) | 0.600 | |
| Dialysis | 508 (14.2%) | 51 (15.6%) | 0.480 | 32 (11.5%) | 41 (14.6%) | 0.266 | |
| Reoperation for bleeding | 504 (14.1%) | 45 (13.8%) | 0.885 | 33 (11.8%) | 37 (13.2%) | 0.609 | |
| Tracheostomy | 291 (8.1%) | 28 (8.6%) | 0.776 | 22 (7.9%) | 22 (7.9%) | 1.000 | |
| Deep sternal wound infection | 79 (2.2%) | 10 (3.1%) | 0.320 | 1 (0.4%) | 10 (3.6%) | 0.006 | |
| Heart failure | 493 (13.8%) | 59 (18.1%) | 0.032 | 35 (12.5%) | 48 (17.1%) | 0.122 | |
| Mechanical circulatory support | 136 (3.8%) | 5 (1.5%) | 0.036 | 8 (2.9%) | 4 (1.4%) | 0.243 | |
| Acute lower limb ischemia | 120 (3.4%) | 4 (1.2%) | 0.031 | 5 (1.8%) | 4 (1.4%) | 1.000 | |
| Major lower limb amputation | 16 (0.4%) | 1 (0.3%) | 1.000 | 0 | 1 (0.4%) | 1.000 | |
| Acute upper limb ischemia | 13 (0.4%) | 0 | 0.618 | 1 (0.4%) | 0 | 1.000 | |
| Surgery for intestinal complications | 17 (0.5%) | 1 (0.3%) | 1.000 | 2 (0.7%) | 1 (0.4%) | 1.000 | |
| 10-year mortality | 1132 (44.0%) | 196 (93.0%) | < 0.001 | 97 (48.0%) | 165 (93.2%) | <0.001 | |
Late Outcome
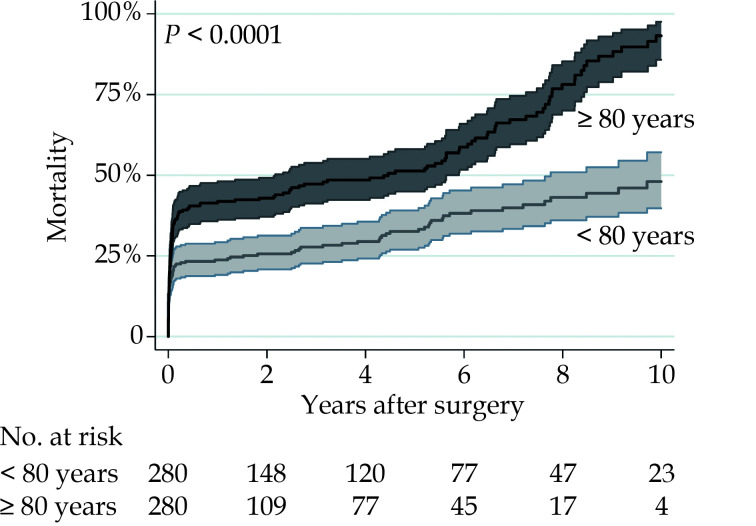
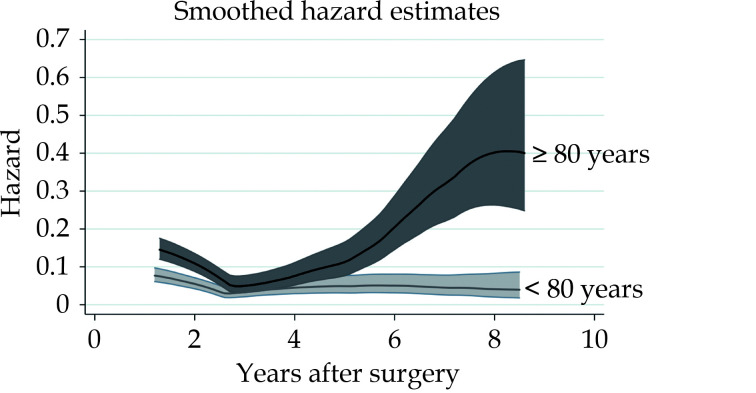
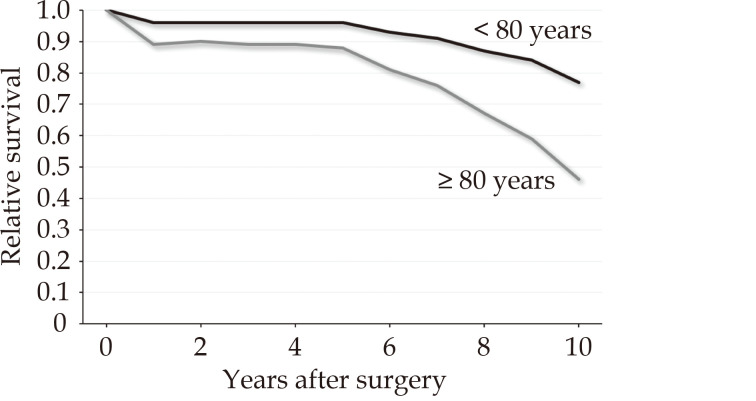
Five-year and 10-year mortality were 51.3% and 93.2% in patients aged ≥ 80 years, and 32.6% and 48.0% in patients aged < 80 years, respectively (P < 0.001) (Figure 1). Smoothed hazard estimates of late mortality in the propensity score matched pairs showed that the hazard of mortality was significantly increased among octogenarians compared to younger patients up to two years after surgery but become comparable to that of younger patients up to 5 years, when hazard of mortality again increased markedly in octogenarians and persisted later (Figure 2). At 5- and 10-year, in the propensity score matched cohorts, the country-, year-, age- and sex-adjusted relative survival were 0.72 (95%CI: 0.65–0.78) and 0.57 (95%CI: 0.46–0.66) among patients aged < 80 years and 0.48 (95%CI: 0.40–0.55) and 0.28 (95%CI: 0.18–0.39) among patients aged ≥ 80 years, respectively. We observed that mortality risk decreased at 3-month after surgery. Based on this observation, we believed reasonable to evaluate the relative survival of patients who survived 3 months after surgery to assess the net benefit after patients’ recovery from surgery. In this subgroup of patients, 5- and 10-year relative survival were 0.96 (95%CI: 0.89–0.99) and 0.77 (95%CI: 0.63–0.86) among patients aged < 80 years, and 0.88 (95%CI: 0.75–0.95) and 0.46 (95%CI: 0.23–0.66) in patients aged ≥ 80 years, respectively (Figure 3). Among octogenarians, relative survival decreased markedly 5 years after surgery (Figure 3).
Figure 1.
Late mortality after surgery for type A aortic dissection in propensity score matched pairs of patients aged < 80 years and ≥ 80 years.
Figure 2.
Hazards of mortality in propensity score matched pairs of patients aged < 80 years and ≥ 80 years.
Figure 3.
Relative survival in propensity score matched pairs of 3-month survivors aged < 80 years and ≥ 80 years.
Predictors of Outcome in Octogenarians
Multilevel mixed-effect logistic regression identified age ≥ 85 years (44.9% vs. 27.4%), preoperative estimated glomerular filtration rate <45 mL/minper 1.73 m2 (44.3% vs. 27.3%), preoperative invasive ventilation (58.5% vs. 27.7%), preoperative mesenteric malperfusion (68.8% vs. 29.7%) and aortic root replacement (57.1% vs. 27.8%) as independent predictors of in-hospital mortality (Table 4). Partial or total aortic arch replacement was also associated with a very high rate of in-hospital mortality compared to younger patients (45.8% vs. 30.5%, P = 0.119), but such a difference did not reach statistical significance likely because the small number of patients (24 patients) who underwent aortic arch surgery among octogenarians. The use of hypothermic circulatory arrest did not increase the risk of in-hospital mortality in the octogenarians (P = 0.607), however tended to increase the risk of stroke/global brain ischemia (P = 0.053). The use of antegrade cerebral perfusion did not have a prognostic effect either on in-hospital mortality (P = 0.277) or stroke/global brain ischemia (P = 0.456).
Table 4. Independent predictors of in-hospital mortality among octogenarians.
| Model A | Model B | |||||
| Model A included eGFR as a continuous variable; Model B included dichotomized eGFR; eGFR: estimated glomerular filtration rate according to the CKD-EPI equation expressed in mL/min per 1.73 m2. | ||||||
| Variables | OR (95%CI) | P-value | Variables | OR (95%CI) | P-value | Additive score |
| Age ≥ 85 yrs | 2.242 (1.303–4.511) | 0.005 | Age ≥ 85 yrs | 2.399 (1.312–4.385) | 0.004 | 1 |
| eGFR | 0.970 (0.956–0.986) | < 0.001 | eGFR < 45 mL/min per 1.73 m2 | 1.904 (1.014–3.572) | 0.045 | 1 |
| Invasive ventilation | 3.806 (1.675–8.650) | 0.001 | Invasive ventilation | 3.801 (1.721–8.396) | 0.001 | 2 |
| Mesenteric malperfusion | 4.758 (1.314–17.226) | 0.017 | Mesenteric malperfusion | 4.145 (1.181–14.555) | 0.026 | 2 |
| Aortic root replacement | 4.325 (1.991–9.395) | < 0.001 | Aortic root replacement | 4.304 (2.001–9.256) | < 0.001 | 2 |
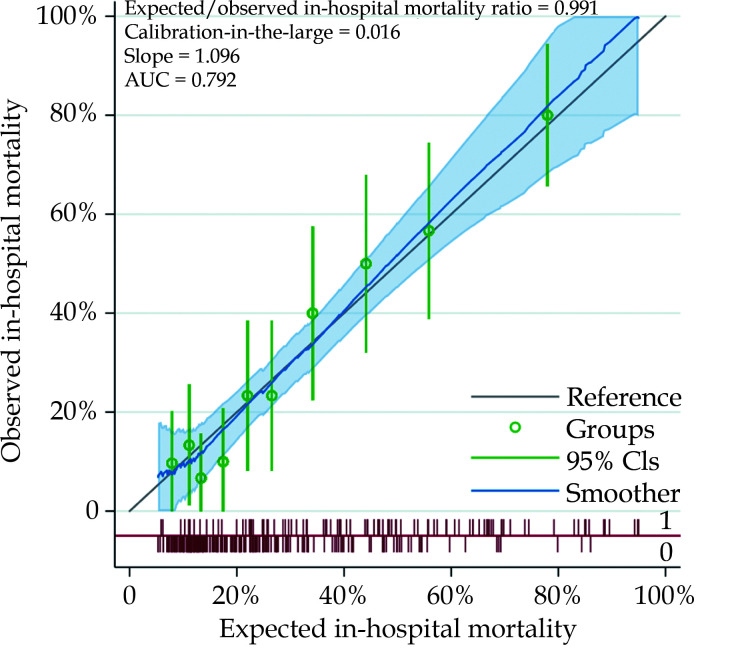
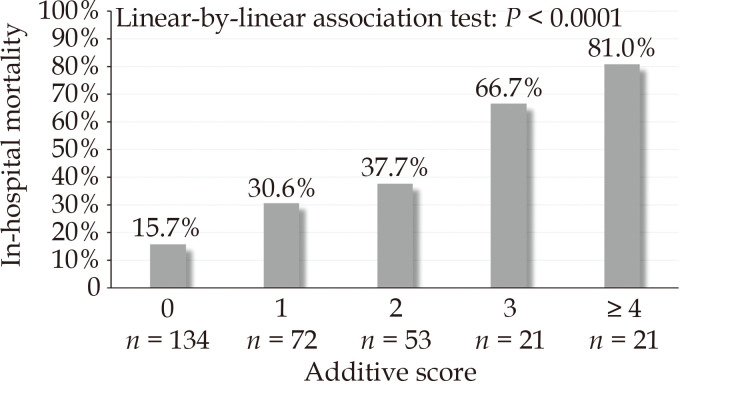
The probabilities of in-hospital mortality estimated by this regression model had an AUC of 0.792 (95%CI: 0.735–0.850) (Hosmer-Lemeshow’s test P = 0.972). The expected/observed in-hospital mortality ratio was 0.991, calibration-in-the-large 0.016, slope 1.096 (Figure 4). Estimated glomerular filtration rate < 45 mL/min per 1.73 m2 was associated with a marked increase of the risk of in-hospital mortality (44.3% vs. 27.3%) and was included into the multivariable regression model for estimation of an additive risk score (Table 4). According to this additive risk score (mean 1.1 ± 1.3) (Table 4), 68.4% of patients had a score ≤ 1 and the in-hospital mortality of these two risk score categories was 20.9%. Markedly increased rates of in-hospital mortality rates were observed in patients with a risk score > 1 (Figure 5). Five-year mortality increased along with this additive risk score (0 point: 42.8%; 1 point: 62.5%; 2 points: 53.0%; 3 points: 73.5%; ≥ 4 points; 94.7%; Log-rank test: P < 0.0001).
Figure 4.
Calibration plot of probabilities of in-hospital mortality in patients aged ≥ 80 years.
Figure 5.
In-hospital mortality rates of patients aged ≥ 80 years according to the additive risk score.
DISCUSSION
The main findings of the present study can be summarized as follows: (1) patients aged ≥ 80 years have a significantly higher risk of in-hospital mortality compared to younger patients; (2) hazard risk and relative survival analyses showed an increased mortality risk among patients aged ≥ 80 years persisting up to 2 years after surgery, but such a risk became comparable to that of younger patients up to 5 years after surgery; (3) relative survival of 3-month survivors was good, but decreased markedly 5 years after surgery both in the elderly and younger patients; (4) surgery for ATAAD can be performed with reasonable low risks of in-hospital and 5-year mortality in patients aged 80–84 years, without compromised kidney function, preoperative invasive mechanical ventilation and/or mesenteric ischemia, and provided that a limited resection of the dissected aorta is performed.
These findings have significant epidemiologic and clinical relevance because of the increasing longevity of the population of the Western countries. When life expectancy was analyzed at the start and the end of the study period of this analysis, we estimated that the median life expectancy of subjects of 80 years of age in the countries participating in this study, had increased from 7.4 years in 2005 to 8.1 years in 2021 among men and from 9.0 years in 2005 to 9.8 years in 2021 among women.[18] This shift in the expectancy of life brings together a higher proportion of very elderly subjects and an increased need of invasive treatments in these patients. The controversy regarding the safety, efficacy and ethical soundness of major surgery is of particular concern when considering the high risk of early postoperative mortality and severe complications in patients with advanced age and the finite nature of human life.
Regarding the clinical outcomes these patients, a recent pooled analysis including data from 16 studies showed that patients aged ≥ 80 years had a significantly higher risk of short-term mortality compared to younger patients (OR = 1.93, 95%CI: 1.33–2.81).[19] However, a significant heterogeneity (I2 = 79.7%) between studies have been observed, with comparable early mortality between the study groups in studies from Japan (OR = 1.14, 95%CI: 0.93–1.40). The important limitation of this analysis resides in the lack of risk adjustment for relevant baseline and operative covariates. In fact, the present study showed that the magnitude of the risk of in-hospital mortality in octogenarians compared to younger patients (crude OR = 2.347, 95%CI: 1.829–3.013; P < 0.001) was mitigated after adjusting differences between the study cohorts with a propensity score matched analysis (adjusted OR = 1.714, 95%CI: 1.162–2.529). A recent study by Park, et al.[21] showed that when adjusted for baseline risk factors, octogenarians may experience comparable outcomes to younger patients. Differences between the studies may be further biased by institutional or individual surgeon’s patient selection policy. Indeed, in the present study, the proportion of octogenarians and their in-hospital mortality differed between the participating hospitals. Consequently, in the present series, a significant number of octogenarians (n = 134, 41%) did not have any risk factor associated with increased in-hospital mortality. It is worth noting that their in-hospital mortality and 5-year mortality were 15.7% and 42.8%, respectively. These results can be considered excellent considering the severity of such a highly lethal condition and the magnitude of emergency aortic surgery in this aged patient population. This observation highlighted the importance of a strict preoperative patients’ selection.
A few studies reported on the late survival of these very elderly. Eranki, et al.[20] pooled the late survival of 472 octogenarians after surgery for ATAAD from seven studies. They estimated that 5-year mortality rate among octogenarians was 46%, a measure which is comparable to the 5-year mortality rate observed in the present study (53.5%).
The risk of most early adverse events was comparable between patients aged ≥ 80 years and their younger counterpart. However, the risk of postoperative stroke was lower in the elderly compared to the younger patients’ cohort. A pooled analysis by Eranki, et al.[20] demonstrated a numerically lower risk of stroke after surgery for ATAAD among octogenarians compared to younger patients (9.4% vs. 10.5%, OR = 0.92, 95%CI: 0.71–1.21). Two large studies reported on numerically lower rates of stroke among octogenarians. Benedetto, et al.[22] reported a postoperative rate of non-fatal cerebrovascular events of 6.4% in octogenarians and 9.4% in younger patients (chi-square test: P = 0.114). Hsu, et al.[23] reported a postoperative rate of stroke of 7.8% in octogenarians and 11.7% in younger patients (chi-square test: P = 0.087). We hypothesize that this finding could be related to a more expedite repair of the thoracic aorta and avoidance of aortic arch replacement in the very elderly.
Regarding the increased risk of deep sternal wound infection in octogenarians, the present study confirmed the knowledge of an association between advanced age and the of risk for postoperative infections after adult cardiac surgery.[24,25]
Maintaining a good quality of life may take priority over lifespan extension.[26] Therefore, the decision to perform major cardiovascular surgery in the elderly should consider the risk of a postoperative decline of the health-related quality in terms of physical, cognitive, and social functioning.[26] Current studies provided controversial results on these issues.[27–31] There are studies reporting comparable postprocedural quality of life among octogenarians and younger patients,[28,29] while other studies observed a significantly poorer quality of life, declining with time, among survivors of surgery for ATAAD compared to the general population.[30,31] The identification of risk factors associated with poor postoperative health-related quality of life is as important as the identification of determinants of postoperative mortality. Future studies on surgery for ATAAD should therefore consider health-related quality of life as one of the main outcome measures.
There are several methodological limitations which should be considered when evaluating the present results. First, the retrospective nature is the main limitation of this study. Second, data on late mortality was retrieved from records of control visits, electronic national registries as well as by contacting regional hospitals, patients and their relatives, but its completeness cannot be verified. Third, patients’ selection might have varied between participating centers as suggested by significant differences in the proportion of octogenarians between hospitals (Table 1). Fourth, the proportion of octogenarians and their in-hospital mortality varied between the hospitals contributing to this multicenter study. However, multilevel mixed-effects regression analyses were performed considering the potential cluster effect of the participating hospitals to address the heterogeneity in the patient’s selection process and operative/perioperative treatment strategies in different institutions. Fifth, we do not have data on patients who were not surgically treated after referral. This prevents a comparative analysis of the baseline characteristics and outcomes of surgically treated patients and of those patients who were left untreated. Finally, the present findings may not apply to non-European population whose risk of early postoperative mortality and severe complications can be much lower.[32]
The strength of the present study resides in the rather large number of patients aged ≥ 80 years and the younger counterpart. Indeed, a post-hoc power analysis showed that a sample size of 123 patients per group would have been enough to reject the null hypothesis of a non-significant difference between in-hospital mortality of the study groups (16.4% vs. 31.6%, alpha 0.005, power 0.80). Survival analysis comparing the observed late mortality of these patients with that of country-, age-, and sex-matched disease-free subjects from the general populations of the participating countries provided a clinically relevant measure of the survival benefit of surgery in these critically ill-patients. Furthermore, this registry gathered data on patients treated at different hospitals from several European countries and the case-mix of this series makes the results generalizable, at least in the Western countries.
Conclusions
Mortality after surgery for ATAAD among patients aged ≥ 80 years is significantly increased compared to younger patients. Still, these results justify emergency surgery in selected octogenarians with ATAAD. In fact, when compared to younger patients and the general population, we observed a satisfactory long-term survival in patients aged ≥ 80 years, and this benefit may last up to 5 years after the procedure. Therefore, clinicians involved in the decision-making process of these very elderly, can be confident to indicate surgical repair of ATAAD in octogenarians with reasonably long-life expectancy, in absence of preoperative mesenteric malperfusion, renal failure, and the need of invasive mechanical ventilation, with the aim to repair only the ascending aorta.
These findings have significant epidemiologic and clinical relevance because of the increasing longevity of the population of the Western countries. Evaluation of the postoperative functional recovery remains of utmost importance[26] and should be investigated to conclusively establish whether surgery for ATAAD is justified in the very elderly.
DECLARATIONS
Trial Registration
ClinicalTrials.gov Identifier: NCT04831073.
Funding Statement
This study was supported by the Finnish Heart Association and by the Sigrid Jusélius Foundation.
Conflict of Interest
Fausto Biancari reports that financial support was provided by the Finnish Heart Association and by the Sigrid Jusélius Foundation, which did not have any role in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the article for publication. The other authors do not have any conflict of interest.
References
- 1.Sisto R Crucial factors affecting longevity. Lancet Healthy Longev. 2023;4:e518–e519. doi: 10.1016/S2666-7568(23)00171-X. [DOI] [PubMed] [Google Scholar]
- 2.Wang J, Chen C, Zhou J, et al Healthy lifestyle in late-life, longevity genes, and life expectancy among older adults: a 20-year, population-based, prospective cohort study. Lancet Healthy Longev. 2023;4:e535–e543. doi: 10.1016/S2666-7568(23)00140-X. [DOI] [PubMed] [Google Scholar]
- 3.Lichtenberg FR The effect of pharmaceutical innovation on longevity: Evidence from the U. S. and 26 high-income countries. Econ Hum Biol. 2022;46:101124. doi: 10.1016/j.ehb.2022.101124. [DOI] [PubMed] [Google Scholar]
- 4.Wang X, Ma H, Li X, et al Association of cardiovascular health with life expectancy free of cardiovascular disease, diabetes, cancer, and dementia in UK adults. JAMA Intern Med. 2023;183:340–349. doi: 10.1001/jamainternmed.2023.0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Groarke JD, Blake G, McCann H, et al Increasing cardiac interventions among the aged. Ir Med J. 2010;103:308–310. [PubMed] [Google Scholar]
- 6.Mäkikallio T, Jalava MP, Husso A, et al Ten-year experience with transcatheter and surgical aortic valve replacement in Finland. Ann Med. 2019;51:270–279. doi: 10.1080/07853890.2019.1614657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sidney S, Go AS, Jaffe MG, et al Association between aging of the US population and heart disease mortality from 2011 to 2017. JAMA Cardiol. 2019;4:1280–1286. doi: 10.1001/jamacardio.2019.4187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Trimarchi S, Eagle KA, Nienaber CA, et al Role of age in acute type A aortic dissection outcome: report from the International Registry of Acute Aortic Dissection (IRAD) J Thorac Cardiovasc Surg. 2010;140:784–789. doi: 10.1016/j.jtcvs.2009.11.014. [DOI] [PubMed] [Google Scholar]
- 9.Biancari F, Vasques F, Benenati V, Juvonen T Contemporary results after surgical repair of type A aortic dissection in patients aged 80 years and older: a systematic review and meta-analysis. Eur J Cardiothorac Surg. 2011;40:1058–1063. doi: 10.1016/j.ejcts.2011.03.044. [DOI] [PubMed] [Google Scholar]
- 10.Kageyama S, Ohashi T, Yoshida T, et al Early mortality of emergency surgery for acute type A aortic dissection in octogenarians and nonagenarians: A multi-center retrospective study. J Thorac Cardiovasc Surg. 2024;167:65–75. doi: 10.1016/j.jtcvs.2022.02.019. [DOI] [PubMed] [Google Scholar]
- 11.Neri E, Toscano T, Massetti M, et al. Operation for acute type A aortic dissection in octogenarians: is it justified? J Thorac Cardiovasc Surg 2001; 121: 259–267.
- 12.Hata M, Sezai A, Niino T, et al. Should emergency surgical intervention be performed for an octogenarian with type A acute aortic dissection? J Thorac Cardiovasc Surg 2008; 135: 1042–1046.
- 13.McKneally MF “We didn't expect dementia and diapers”: reflections on the Nihon experience with type A aortic dissection in octogenarians. J Thorac Cardiovasc Surg. 2008;135:984–985. doi: 10.1016/j.jtcvs.2007.10.068. [DOI] [PubMed] [Google Scholar]
- 14.Biancari F, Mariscalco G, Yusuff H, et al European Registry of Type A Aortic Dissection (ERTAAD) - rationale, design and definition criteria. J Cardiothorac Surg. 2021;16:171. doi: 10.1186/s13019-021-01536-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Levey AS, Stevens LA, Schmid CH, et al A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Estève J, Benhamou E, Croasdale M, Raymond L Relative survival and the estimation of net survival: elements for further discussion. Stat Med. 1990;9:529–538. doi: 10.1002/sim.4780090506. [DOI] [PubMed] [Google Scholar]
- 17.Hills M, Rachet B, Falcaro M Strel2: A command for estimating excess hazard and relative survival in large population-based studies. Stata J. 2014;14:176–190. doi: 10.1177/1536867X1401400112. [DOI] [Google Scholar]
- 18.Human Mortality Database. (Accessed January 7, 2024 at https://www.mortality.org/).
- 19.Ensor J, Snell KIE, Martin EC. PMCALPLOT: Stata module to produce calibration plot of prediction model performance. Statistical Software Components S458486, Boston College Department of Economics, 2018.
- 20.Eranki A, Merakis M, Williams ML, et al Outcomes of surgery for acute type A dissection in octogenarians versus non-octogenarians: a systematic review and meta-analysis. J Cardiothorac Surg. 2022;17:222. doi: 10.1186/s13019-022-01980-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Park YK, Lee JH, Kim KM, et al Acute type A aortic dissection features and outcomes in octogenarians: a propensity score analysis. Interdiscip Cardiovasc Thorac Surg. 2024;38:ivae038. doi: 10.1093/icvts/ivae038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Benedetto U, Sinha S, Dimagli A, et al Decade-long trends in surgery for acute Type A aortic dissection in England: A retrospective cohort study. Lancet Reg Health Eur. 2021;7:100131. doi: 10.1016/j.lanepe.2021.100131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hsu ME, Chou AH, Cheng YT, et al Outcomes of acute aortic dissection surgery in octogenarians. J Am Heart Assoc. 2020;9:e017147. doi: 10.1161/JAHA.120.017147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Borger MA, Rao V, Weisel RD, et al Deep sternal wound infection: risk factors and outcomes. Ann Thorac Surg. 1998;65:1050–1056. doi: 10.1016/S0003-4975(98)00063-0. [DOI] [PubMed] [Google Scholar]
- 25.Fowler VG Jr, O'Brien SM, Muhlbaier LH, et al. Clinical predictors of major infections after cardiac surgery. Circulation 2005; 112(9 Suppl): I358–I365.
- 26.Harky A, Bailey G, Othman A, et al The life in their years versus the years in their life. J Thorac Cardiovasc Surg. 2021;161:e361–e362. doi: 10.1016/j.jtcvs.2020.11.113. [DOI] [PubMed] [Google Scholar]
- 27.Breel JS, de Klerk ES, Strypet M, et al What really matters to survivors of acute type A aortic dissection-A survey of patient-reported outcomes in the Dutch National Aortic Dissection Advocacy Group. J Clin Med. 2023;12:6584. doi: 10.3390/jcm12206584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bojko MM, Suhail M, Bavaria JE, et al Midterm outcomes of emergency surgery for acute type A aortic dissection in octogenarians. J Thorac Cardiovasc Surg. 2022;163:2–12. doi: 10.1016/j.jtcvs.2020.03.157. [DOI] [PubMed] [Google Scholar]
- 29.Tang GH, Malekan R, Yu CJ, et al. Surgery for acute type A aortic dissection in octogenarians is justified. J Thorac Cardiovasc Surg 2013; 145(3 Suppl): S186–S190.
- 30.Adam U, Habazettl H, Graefe K, et al Health-related quality of life of patients after surgery for acute type A aortic dissection. Interact Cardiovasc Thorac Surg. 2018;27:48–53. doi: 10.1093/icvts/ivy036. [DOI] [PubMed] [Google Scholar]
- 31.Endlich M, Hamiko M, Gestrich C, et al Long-term outcome and quality of life in aortic type A dissection survivors. Thorac Cardiovasc Surg. 2016;64:91–99. doi: 10.1055/s-0035-1548734. [DOI] [PubMed] [Google Scholar]
- 32.Zhao R, Qiu J, Dai L, et al Current surgical management of acute type A aortic dissection in China: a multicenter registry study. JACC Asia. 2022;2:869–878. doi: 10.1016/j.jacasi.2022.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]