Abstract
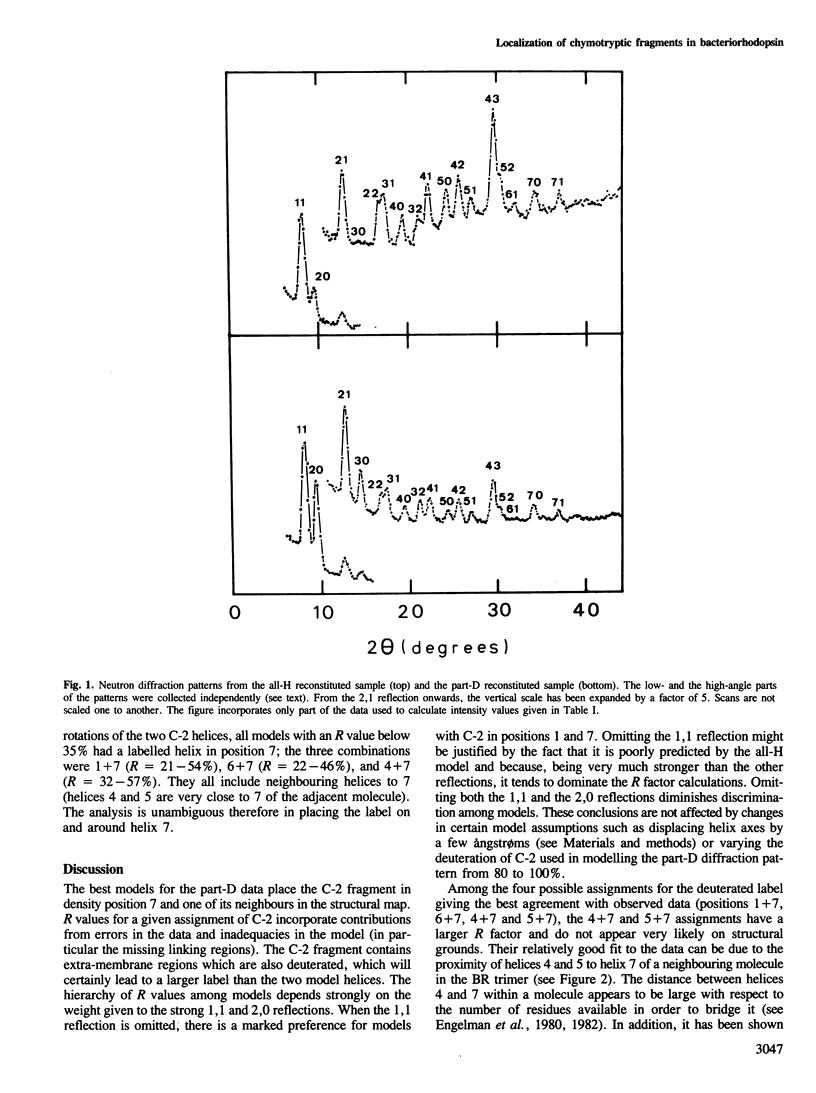
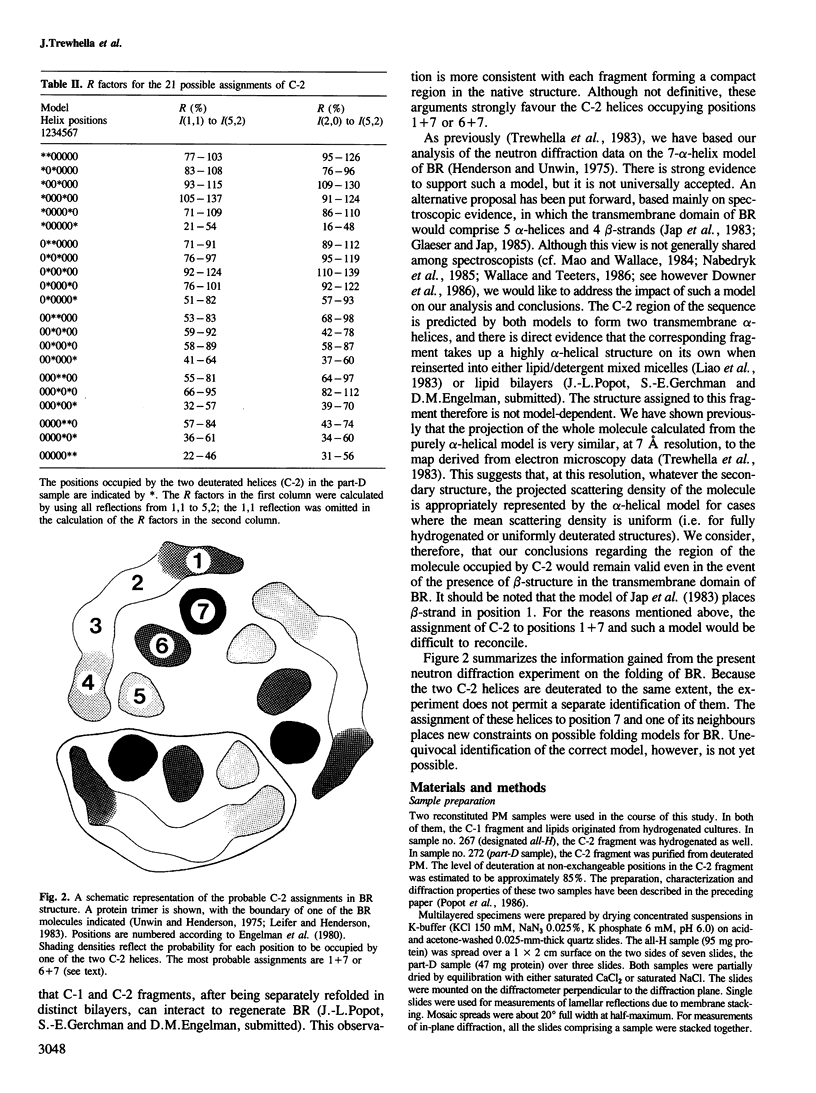
The structure of crystalline purple membrane reconstituted from purified bacteriorhodopsin (BR) chymotryptic fragments has been studied by neutron diffraction. In one of the samples studied, the fragment C-2, encompassing the first two predicted transmembrane segments, was prepared from deuterated purple membrane. The diffraction changes when the natural C-2 fragment is substituted by a deuterated one are analysed in terms of a seven-helix model for BR. The assignment of the labelled fragment to one end of the molecule placed new constraints on folding models for the protein.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agard D. A., Stroud R. M. Linking regions between helices in bacteriorhodopsin revealed. Biophys J. 1982 Mar;37(3):589–602. [PMC free article] [PubMed] [Google Scholar]
- Downer N. W., Bruchman T. J., Hazzard J. H. Infrared spectroscopic study of photoreceptor membrane and purple membrane. Protein secondary structure and hydrogen deuterium exchange. J Biol Chem. 1986 Mar 15;261(8):3640–3647. [PubMed] [Google Scholar]
- Dumont M. E., Trewhella J., Engelman D. M., Richards F. M. Stability of transmembrane regions in bacteriorhodopsin studied by progressive proteolysis. J Membr Biol. 1985;88(3):233–247. doi: 10.1007/BF01871088. [DOI] [PubMed] [Google Scholar]
- Engelman D. M., Henderson R., McLachlan A. D., Wallace B. A. Path of the polypeptide in bacteriorhodopsin. Proc Natl Acad Sci U S A. 1980 Apr;77(4):2023–2027. doi: 10.1073/pnas.77.4.2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelman D. M., Zaccai G. Bacteriorhodopsin is an inside-out protein. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5894–5898. doi: 10.1073/pnas.77.10.5894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaeser R. M., Jap B. K. Absorption flattening in the circular dichroism spectra of small membrane fragments. Biochemistry. 1985 Nov 5;24(23):6398–6401. doi: 10.1021/bi00344a012. [DOI] [PubMed] [Google Scholar]
- Henderson R., Unwin P. N. Three-dimensional model of purple membrane obtained by electron microscopy. Nature. 1975 Sep 4;257(5521):28–32. doi: 10.1038/257028a0. [DOI] [PubMed] [Google Scholar]
- Huang K. S., Radhakrishnan R., Bayley H., Khorana H. G. Orientation of retinal in bacteriorhodopsin as studied by cross-linking using a photosensitive analog of retinal. J Biol Chem. 1982 Nov 25;257(22):13616–13623. [PubMed] [Google Scholar]
- Jap B. K., Maestre M. F., Hayward S. B., Glaeser R. M. Peptide-chain secondary structure of bacteriorhodopsin. Biophys J. 1983 Jul;43(1):81–89. doi: 10.1016/S0006-3495(83)84326-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katre N. V., Finer-Moore J., Stroud R. M., Hayward S. B. Location of an extrinsic label in the primary and tertiary structure of bacteriorhodopsin. Biophys J. 1984 Aug;46(2):195–203. doi: 10.1016/S0006-3495(84)84013-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khorana H. G., Gerber G. E., Herlihy W. C., Gray C. P., Anderegg R. J., Nihei K., Biemann K. Amino acid sequence of bacteriorhodopsin. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5046–5050. doi: 10.1073/pnas.76.10.5046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leifer D., Henderson R. Three-dimensional structure of orthorhombic purple membrane at 6.5 A resolution. J Mol Biol. 1983 Jan 25;163(3):451–466. doi: 10.1016/0022-2836(83)90068-2. [DOI] [PubMed] [Google Scholar]
- Liao M. J., London E., Khorana H. G. Regeneration of the native bacteriorhodopsin structure from two chymotryptic fragments. J Biol Chem. 1983 Aug 25;258(16):9949–9955. [PubMed] [Google Scholar]
- Mao D., Wallace B. A. Differential light scattering and absorption flattening optical effects are minimal in the circular dichroism spectra of small unilamellar vesicles. Biochemistry. 1984 Jun 5;23(12):2667–2673. doi: 10.1021/bi00307a020. [DOI] [PubMed] [Google Scholar]
- Nabedryk E., Bardin A. M., Breton J. Further characterization of protein secondary structures in purple membrane by circular dichroism and polarized infrared spectroscopies. Biophys J. 1985 Dec;48(6):873–876. doi: 10.1016/S0006-3495(85)83848-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ovchinnikov YuA Rhodopsin and bacteriorhodopsin: structure-function relationships. FEBS Lett. 1982 Nov 8;148(2):179–191. doi: 10.1016/0014-5793(82)80805-3. [DOI] [PubMed] [Google Scholar]
- Ovchinnikov Y. A., Abdulaev N. G., Feigina M. Y., Kiselev A. V., Lobanov N. A. The structural basis of the functioning of bacteriorhodopsin: an overview. FEBS Lett. 1979 Apr 15;100(2):219–224. doi: 10.1016/0014-5793(79)80338-5. [DOI] [PubMed] [Google Scholar]
- Popot J. L., Trewhella J., Engelman D. M. Reformation of crystalline purple membrane from purified bacteriorhodopsin fragments. EMBO J. 1986 Nov;5(11):3039–3044. doi: 10.1002/j.1460-2075.1986.tb04603.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogan P. K., Zaccai G. Hydration in purple membrane as a function of relative humidity. J Mol Biol. 1981 Jan 5;145(1):281–284. doi: 10.1016/0022-2836(81)90344-2. [DOI] [PubMed] [Google Scholar]
- Seiff F., Wallat I., Ermann P., Heyn M. P. A neutron diffraction study on the location of the polyene chain of retinal in bacteriorhodopsin. Proc Natl Acad Sci U S A. 1985 May;82(10):3227–3231. doi: 10.1073/pnas.82.10.3227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoeckenius W., Bogomolni R. A. Bacteriorhodopsin and related pigments of halobacteria. Annu Rev Biochem. 1982;51:587–616. doi: 10.1146/annurev.bi.51.070182.003103. [DOI] [PubMed] [Google Scholar]
- Trewhella J., Anderson S., Fox R., Gogol E., Khan S., Engelman D., Zaccai G. Assignment of segments of the bacteriorhodopsin sequence to positions in the structural map. Biophys J. 1983 Jun;42(3):233–241. doi: 10.1016/S0006-3495(83)84391-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unwin P. N., Henderson R. Molecular structure determination by electron microscopy of unstained crystalline specimens. J Mol Biol. 1975 May 25;94(3):425–440. doi: 10.1016/0022-2836(75)90212-0. [DOI] [PubMed] [Google Scholar]
- Wallace B. A., Henderson R. Location of the carboxyl terminus of bacteriorhodopsin in purple membrane. Biophys J. 1982 Sep;39(3):233–239. doi: 10.1016/S0006-3495(82)84513-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaccai G., Gilmore D. J. Areas of hydration in the purple membrane of Halobacterium halobium: a neutron diffraction study. J Mol Biol. 1979 Aug 5;132(2):181–191. doi: 10.1016/0022-2836(79)90390-5. [DOI] [PubMed] [Google Scholar]


