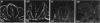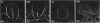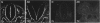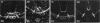Abstract
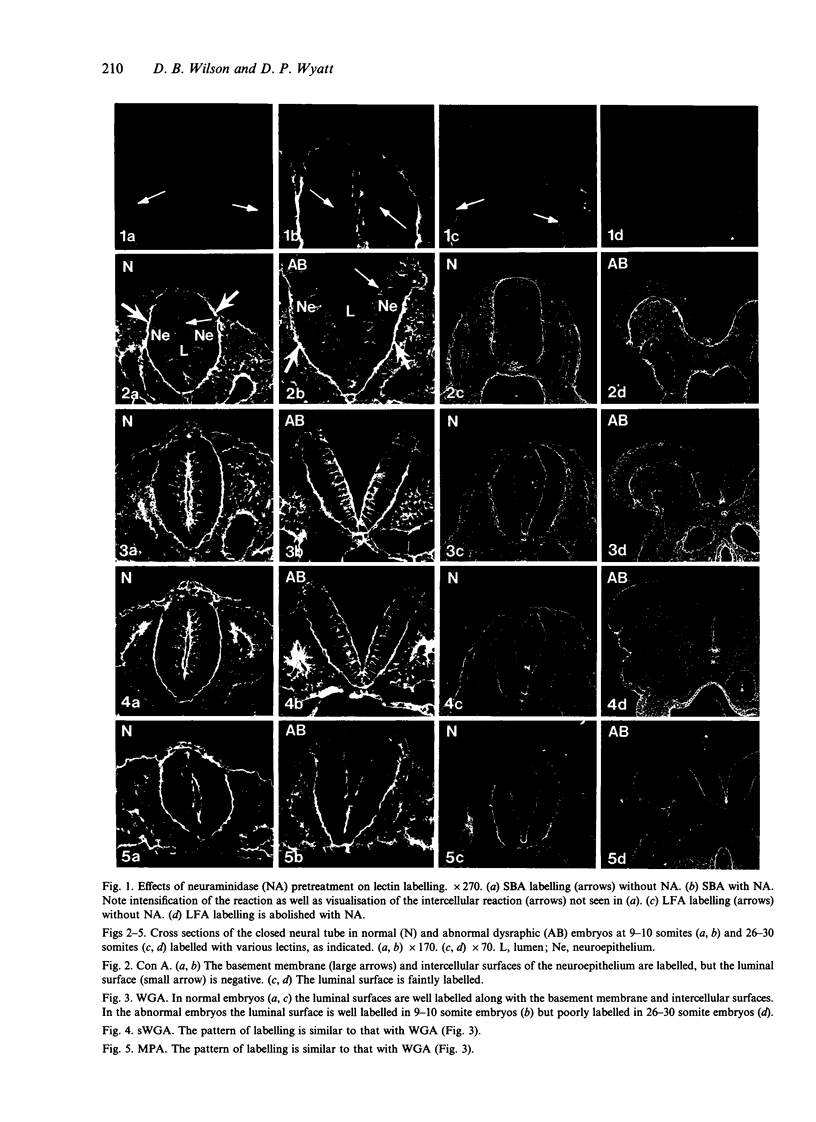
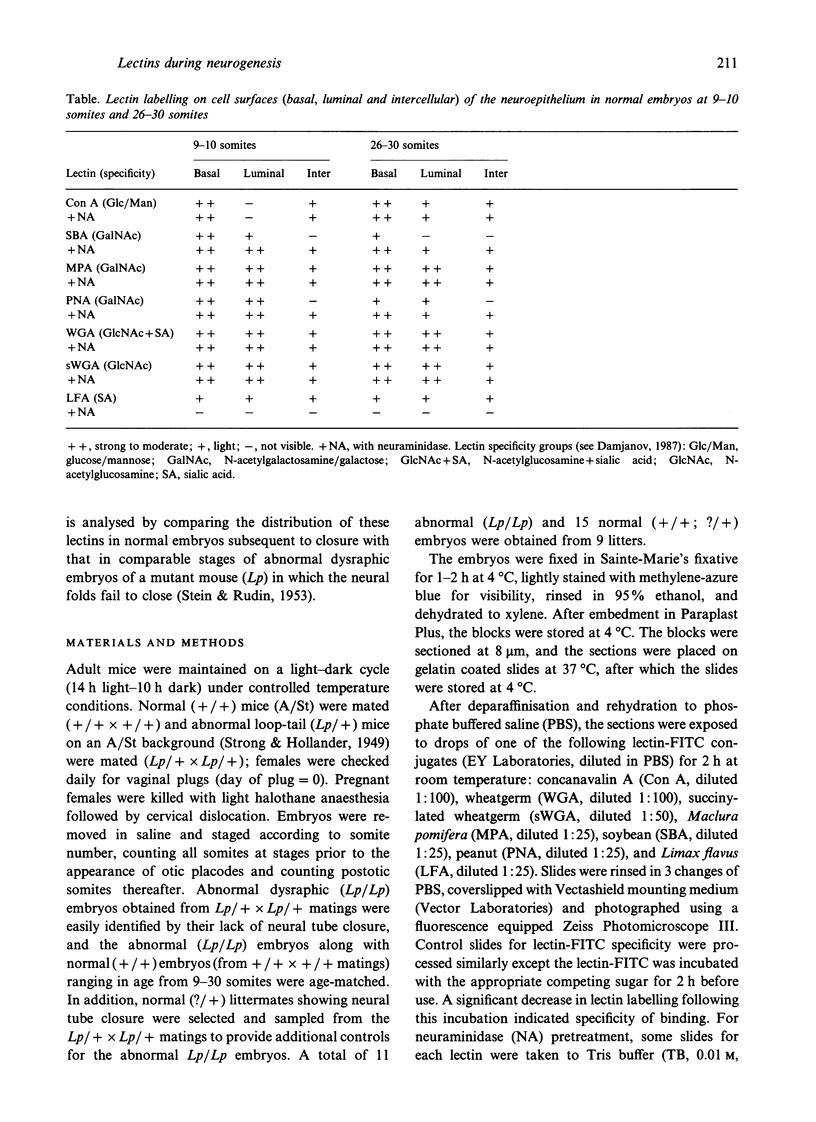
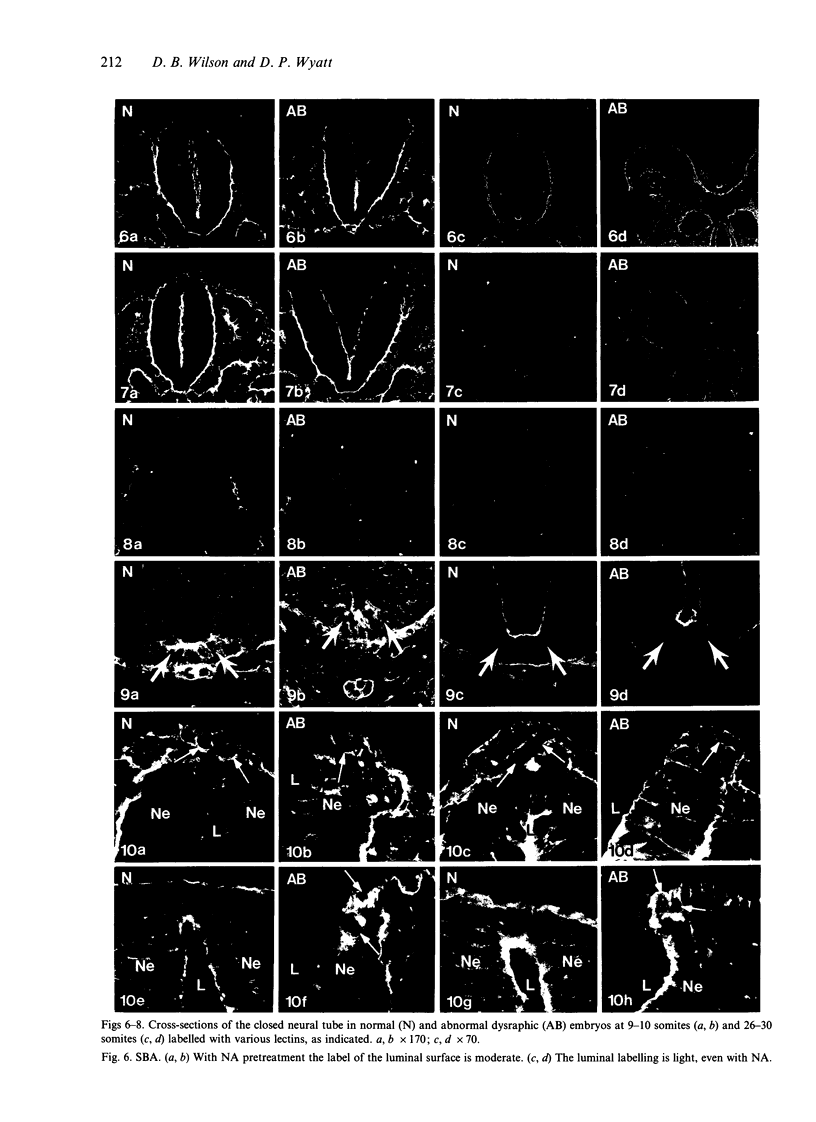
Temporospatial changes in surface carbohydrates of neuroepithelial cells were analysed by means of lectin histochemistry in normal mouse embryos subsequent to closure of the neural tube. The lectins used were concanavalin A (con A), soybean (SBA), Maclura pomifera (MPA), peanut (PNA), wheatgerm (WGA), succinylated wheatgerm (sWGA) and Limax flavus (LFA). Although labelling was obtained with all of the lectins, the most striking temporospatial differences occurred with con A which in the early embryos (9-10 somites) labelled the basal and intercellular surfaces, but not the luminal surfaces of the neuroepithelial cells, whereas in the older embryos (26-30 somites), con A showed light luminal surface labelling. A midventral wedge of cells in the floor of the neural tube in the older embryos also exhibited more intense labelling with con A, WGA, and sWGA than with the other lectins. In addition, comparisons of lectin localisation were made between the closed neural tube in normal embryos and the open neural folds in the loop-tail (Lp) mutant mouse in which the neural tube fails to close. Although similar temporospatial patterns in lectin localisation occurred as in normal embryos, the retention of lectin labelling associated with rounded putative neural crest cells that remained sequestered in the apices of the open neural folds, along with an attenuation of the luminal reaction in the older abnormal embryos, suggest that during normal mammalian development closure of the spinal neural folds may be important for the timely exit of neural crest cells as well as for eliciting changes in the luminal surfaces of the neuroepithelial cells.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adam E., Dziegielewska K. M., Saunders N. R., Schumacher U. Neuraminic acid specific lectins as markers of early cortical plate neurons. Int J Dev Neurosci. 1993 Aug;11(4):451–460. doi: 10.1016/0736-5748(93)90019-a. [DOI] [PubMed] [Google Scholar]
- Bush K. T., Lynch F. J., DeNittis A. S., Steinberg A. B., Lee H. Y., Nagele R. G. Neural tube formation in the mouse: a morphometric and computerized three-dimensional reconstruction study of the relationship between apical constriction of neuroepithelial cells and the shape of the neuroepithelium. Anat Embryol (Berl) 1990;181(1):49–58. doi: 10.1007/BF00189727. [DOI] [PubMed] [Google Scholar]
- Currie J. R., Maylié-Pfenninger M. F., Pfenninger K. H. Developmentally regulated plasmalemmal glycoconjugates of the surface and neural ectoderm. Dev Biol. 1984 Nov;106(1):109–120. doi: 10.1016/0012-1606(84)90067-8. [DOI] [PubMed] [Google Scholar]
- Damjanov I., Black P. Lectin binding sites on the luminal surface of ependymal cells of the rat spinal cord: implications for neuropathological investigation. Neurosurgery. 1987 May;20(5):722–725. doi: 10.1227/00006123-198705000-00008. [DOI] [PubMed] [Google Scholar]
- Damjanov I. Lectin cytochemistry and histochemistry. Lab Invest. 1987 Jul;57(1):5–20. [PubMed] [Google Scholar]
- DeGrauw T. J., Liwnicz B. H. Lectins are markers of neuronal migration and differentiation in rat brain. Dev Neurosci. 1986;8(4):236–242. doi: 10.1159/000112257. [DOI] [PubMed] [Google Scholar]
- Erickson C. A., Weston J. A. An SEM analysis of neural crest migration in the mouse. J Embryol Exp Morphol. 1983 Apr;74:97–118. [PubMed] [Google Scholar]
- Gato A., Moro J. A., Alonso M. I., Pastor J. F., Represa J. J., Barbosa E. Chondroitin sulphate proteoglycan and embryonic brain enlargement in the chick. Anat Embryol (Berl) 1993 Jul;188(1):101–106. doi: 10.1007/BF00191455. [DOI] [PubMed] [Google Scholar]
- Griffith C. M., Sanders E. J. Changes in glycoconjugate expression during early chick embryo development: a lectin-binding study. Anat Rec. 1991 Oct;231(2):238–250. doi: 10.1002/ar.1092310212. [DOI] [PubMed] [Google Scholar]
- Griffith C. M., Wiley M. J. The distribution of cell surface glycoconjugates during mouse secondary neurulation. Anat Embryol (Berl) 1989;180(6):567–575. doi: 10.1007/BF00300554. [DOI] [PubMed] [Google Scholar]
- Hoving E. W., Vermeij-Keers C., Mommaas-Kienhuis A. M., Hartwig N. G. Separation of neural and surface ectoderm after closure of the rostral neuropore. Anat Embryol (Berl) 1990;182(5):455–463. doi: 10.1007/BF00178910. [DOI] [PubMed] [Google Scholar]
- Innes P. B. The ultrastructure of early cephalic neural crest cell migration in the mouse. Anat Embryol (Berl) 1985;172(1):33–38. doi: 10.1007/BF00318941. [DOI] [PubMed] [Google Scholar]
- Layer P. G., Alber R. Patterning of chick brain vesicles as revealed by peanut agglutinin and cholinesterases. Development. 1990 Jul;109(3):613–624. doi: 10.1242/dev.109.3.613. [DOI] [PubMed] [Google Scholar]
- Moase C. E., Trasler D. G. Delayed neural crest cell emigration from Sp and Spd mouse neural tube explants. Teratology. 1990 Aug;42(2):171–182. doi: 10.1002/tera.1420420208. [DOI] [PubMed] [Google Scholar]
- Nichols D. H. Ultrastructure of neural crest formation in the midbrain/rostral hindbrain and preotic hindbrain regions of the mouse embryo. Am J Anat. 1987 Jun;179(2):143–154. doi: 10.1002/aja.1001790207. [DOI] [PubMed] [Google Scholar]
- Nieto M. A., Sargent M. G., Wilkinson D. G., Cooke J. Control of cell behavior during vertebrate development by Slug, a zinc finger gene. Science. 1994 May 6;264(5160):835–839. doi: 10.1126/science.7513443. [DOI] [PubMed] [Google Scholar]
- Placzek M., Jessell T. M., Dodd J. Induction of floor plate differentiation by contact-dependent, homeogenetic signals. Development. 1993 Jan;117(1):205–218. doi: 10.1242/dev.117.1.205. [DOI] [PubMed] [Google Scholar]
- Placzek M., Yamada T., Tessier-Lavigne M., Jessell T., Dodd J. Control of dorsoventral pattern in vertebrate neural development: induction and polarizing properties of the floor plate. Dev Suppl. 1991;Suppl 2:105–122. [PubMed] [Google Scholar]
- Sanders E. J. Cytochemistry of the cell surface and extracellular matrix during early embryonic development. Prog Histochem Cytochem. 1986;16(3):1–57. doi: 10.1016/s0079-6336(86)80001-8. [DOI] [PubMed] [Google Scholar]
- Sato M., Yonezawa S., Uehara H., Arita Y., Sato E., Muramatsu T. Differential distribution of receptors for two fucose-binding lectins in embryos and adult tissues of the mouse. Differentiation. 1986;30(3):211–219. doi: 10.1111/j.1432-0436.1986.tb00783.x. [DOI] [PubMed] [Google Scholar]
- Schoenwolf G. C., Franks M. V. Quantitative analyses of changes in cell shapes during bending of the avian neural plate. Dev Biol. 1984 Oct;105(2):257–272. doi: 10.1016/0012-1606(84)90284-7. [DOI] [PubMed] [Google Scholar]
- Schoenwolf G. C. Shaping and bending of the avian neuroepithelium: morphometric analyses. Dev Biol. 1985 May;109(1):127–139. doi: 10.1016/0012-1606(85)90353-7. [DOI] [PubMed] [Google Scholar]
- Schoenwolf G. C., Smith J. L. Mechanisms of neurulation: traditional viewpoint and recent advances. Development. 1990 Jun;109(2):243–270. doi: 10.1242/dev.109.2.243. [DOI] [PubMed] [Google Scholar]
- Shepard T. H., Park H. W., Pascoe-Mason J. Glucose causes lengthening of the microvilli of the neural plate of the rat embryo and produces a helical pattern on their surface. Teratology. 1993 Jul;48(1):65–74. doi: 10.1002/tera.1420480111. [DOI] [PubMed] [Google Scholar]
- Smith J. C. Dorso-ventral patterning in the neural tube. Curr Biol. 1993 Sep 1;3(9):582–585. doi: 10.1016/0960-9822(93)90003-7. [DOI] [PubMed] [Google Scholar]
- Smith J. L., Schoenwolf G. C. Notochordal induction of cell wedging in the chick neural plate and its role in neural tube formation. J Exp Zool. 1989 Apr;250(1):49–62. doi: 10.1002/jez.1402500107. [DOI] [PubMed] [Google Scholar]
- Smits-van Prooije A. E., Poelmann R. E., Gesink A. F., van Groeningen M. J., Vermeij-Keers C. The cell surface coat in neurulating mouse and rat embryos, studied with lectins. Anat Embryol (Berl) 1986;175(1):111–117. doi: 10.1007/BF00315461. [DOI] [PubMed] [Google Scholar]
- Sternberg J., Kimber S. J. Distribution of fibronectin, laminin and entactin in the environment of migrating neural crest cells in early mouse embryos. J Embryol Exp Morphol. 1986 Feb;91:267–282. [PubMed] [Google Scholar]
- Sternberg J., Kimber S. J. The relationship between emerging neural crest cells and basement membranes in the trunk of the mouse embryo: a TEM and immunocytochemical study. J Embryol Exp Morphol. 1986 Nov;98:251–268. [PubMed] [Google Scholar]
- Takahashi H. Changes in peanut lectin binding sites on the neuroectoderm during neural tube formation in the bantam chick embryo. Anat Embryol (Berl) 1988;178(4):353–358. doi: 10.1007/BF00698666. [DOI] [PubMed] [Google Scholar]
- Takahashi H., Howes R. I. Binding pattern of ferritin-labeled lectins (RCAI and WGA) during neural tube closure in the bantam embryo. Anat Embryol (Berl) 1986;174(3):283–288. doi: 10.1007/BF00698778. [DOI] [PubMed] [Google Scholar]
- Takahashi H. The masking effect of sialic acid on Con A, PNA and SBA ectoderm binding sites during neurulation in the bantam chick embryo. Anat Embryol (Berl) 1992;185(4):389–400. doi: 10.1007/BF00188550. [DOI] [PubMed] [Google Scholar]
- Wilson D. B., Finta L. A. Early development of the brain and spinal cord in dysraphic mice. Anat Embryol (Berl) 1980;160(3):315–326. doi: 10.1007/BF00305111. [DOI] [PubMed] [Google Scholar]
- Wilson D. B., Finta L. A. Early development of the brain and spinal cord in dysraphic mice: a transmission electron microscopic study. J Comp Neurol. 1980 Mar 15;190(2):363–371. doi: 10.1002/cne.901900210. [DOI] [PubMed] [Google Scholar]
- Wilson D. B., Wyatt D. P. Abnormal elevation of the neural folds in the loop-tail mutant mouse. Acta Anat (Basel) 1992;143(2):89–95. doi: 10.1159/000147234. [DOI] [PubMed] [Google Scholar]
- Wilson D. B., Wyatt D. P. Ultrastructural defects in the apical neural folds in mutant embryos with spina bifida. Acta Neuropathol. 1989;79(1):94–100. doi: 10.1007/BF00308963. [DOI] [PubMed] [Google Scholar]
- Yamada T., Placzek M., Tanaka H., Dodd J., Jessell T. M. Control of cell pattern in the developing nervous system: polarizing activity of the floor plate and notochord. Cell. 1991 Feb 8;64(3):635–647. doi: 10.1016/0092-8674(91)90247-v. [DOI] [PubMed] [Google Scholar]