Abstract
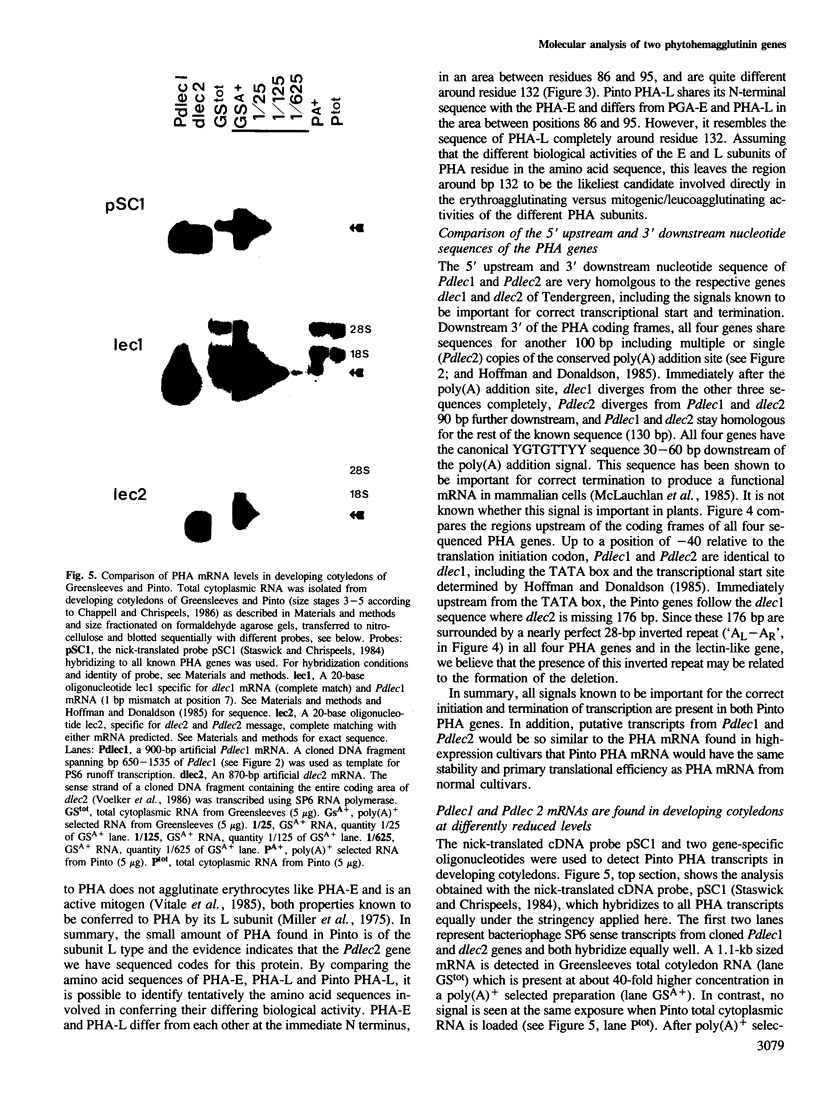
Phytohemagglutinin (PHA), the seed lectin of the common bean, Phaseolus vulgaris, is encoded by two highly homologous, tandemly linked genes, dlec1 and dlec2, which are coordinately expressed at high levels in developing cotyledons. Their respective transcripts translate into closely related polypeptides, PHA-E and PHA-L, constituents of the tetrameric lectin which accumulates at high levels in developing seeds. In the bean cultivar Pinto UI111, PHA-E is not detectable, and PHA-L accumulates at very reduced levels. To investigate the cause of the Pinto phenotype, we cloned and sequenced the two PHA genes of Pinto, called Pdlec1 and Pdlec2, and determined the abundance of their respective mRNAs in developing cotyledons. Both genes are more than 90% homologous to the normal PHA genes found in other cultivars. Pdlec1 carries a 1-bp frameshift mutation close to the 5' end of its coding sequence. Only very truncated polypeptides could be made from its mRNA. The gene Pdlec2 encodes a polypeptide, which resembles PHA-L and its predicted amino acid sequence agrees with the available Pinto PHA amino acid sequence data. Analysis of the mRNA of developing cotyledons revealed that the Pdlec1 message is reduced 600-fold, and Pdlec2 mRNA is reduced 20-fold with respect to mRNA levels in normal cultivars. A comparison of the sequences which are upstream from the coding sequence shows that Pdlec2 has a 100-bp deletion compared to the other genes (dlec1, dlec2 and Pdlec1). This deletion which contains a large tandem repeat may be responsible for the low level of expression of Pdlec2. The very low expression of Pdlec1 is as yet unexplained.
Keywords: phytohemagglutinin genes, Phaseolus vulgaris, dlec1 and 2, Pdlec1 and 2
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bown D., Levasseur M., Croy R. R., Boulter D., Gatehouse J. A. Sequence of a pseudogene in the legumin gene family of pea (Pisum sativum L.). Nucleic Acids Res. 1985 Jun 25;13(12):4527–4538. doi: 10.1093/nar/13.12.4527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand A. H., Breeden L., Abraham J., Sternglanz R., Nasmyth K. Characterization of a "silencer" in yeast: a DNA sequence with properties opposite to those of a transcriptional enhancer. Cell. 1985 May;41(1):41–48. doi: 10.1016/0092-8674(85)90059-5. [DOI] [PubMed] [Google Scholar]
- Chappell J., Chrispeels M. J. Transcriptional and Posttranscriptional Control of Phaseolin and Phytohemagglutinin Gene Expression in Developing Cotyledons of Phaseolus vulgaris. Plant Physiol. 1986 May;81(1):50–54. doi: 10.1104/pp.81.1.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle J. J., Schuler M. A., Godette W. D., Zenger V., Beachy R. N., Slightom J. L. The glycosylated seed storage proteins of Glycine max and Phaseolus vulgaris. Structural homologies of genes and proteins. J Biol Chem. 1986 Jul 15;261(20):9228–9238. [PubMed] [Google Scholar]
- Forman M. R., Graubard B. I., Hoffman H. J., Beren R., Harley E. E., Bennett P. The Pima infant feeding study: breastfeeding and respiratory infections during the first year of life. Int J Epidemiol. 1984 Dec;13(4):447–453. doi: 10.1093/ije/13.4.447. [DOI] [PubMed] [Google Scholar]
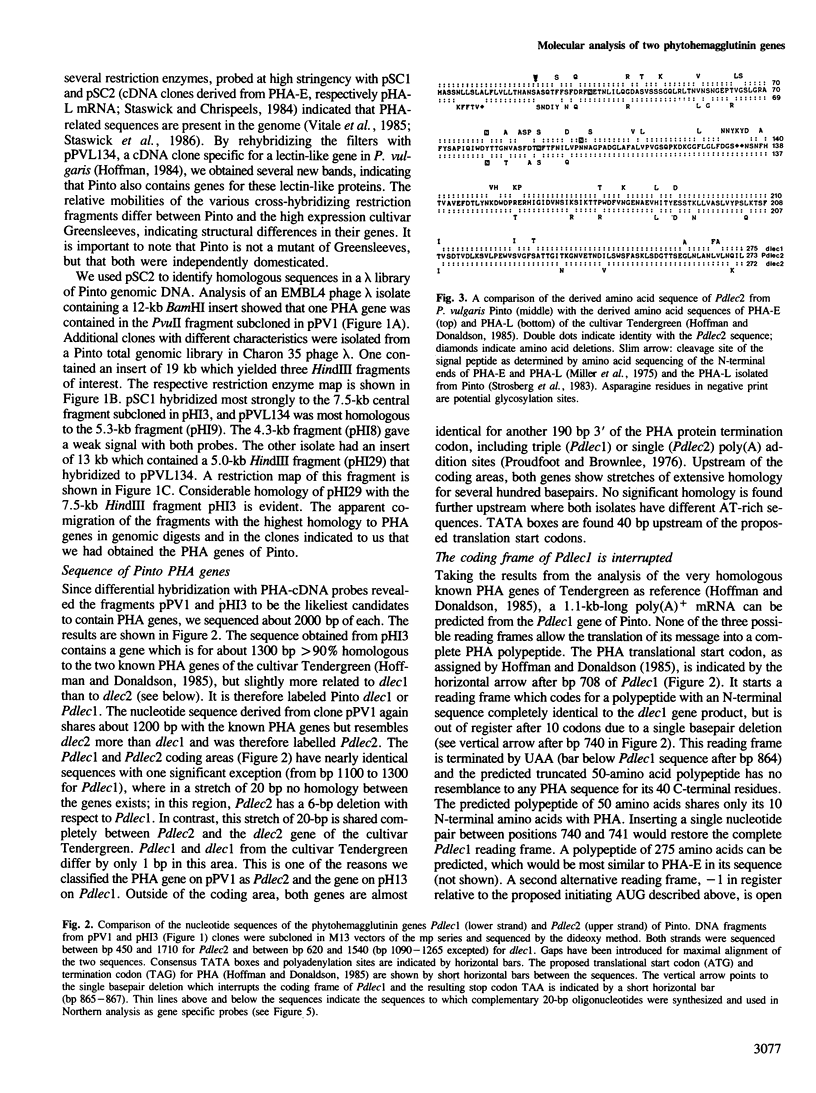
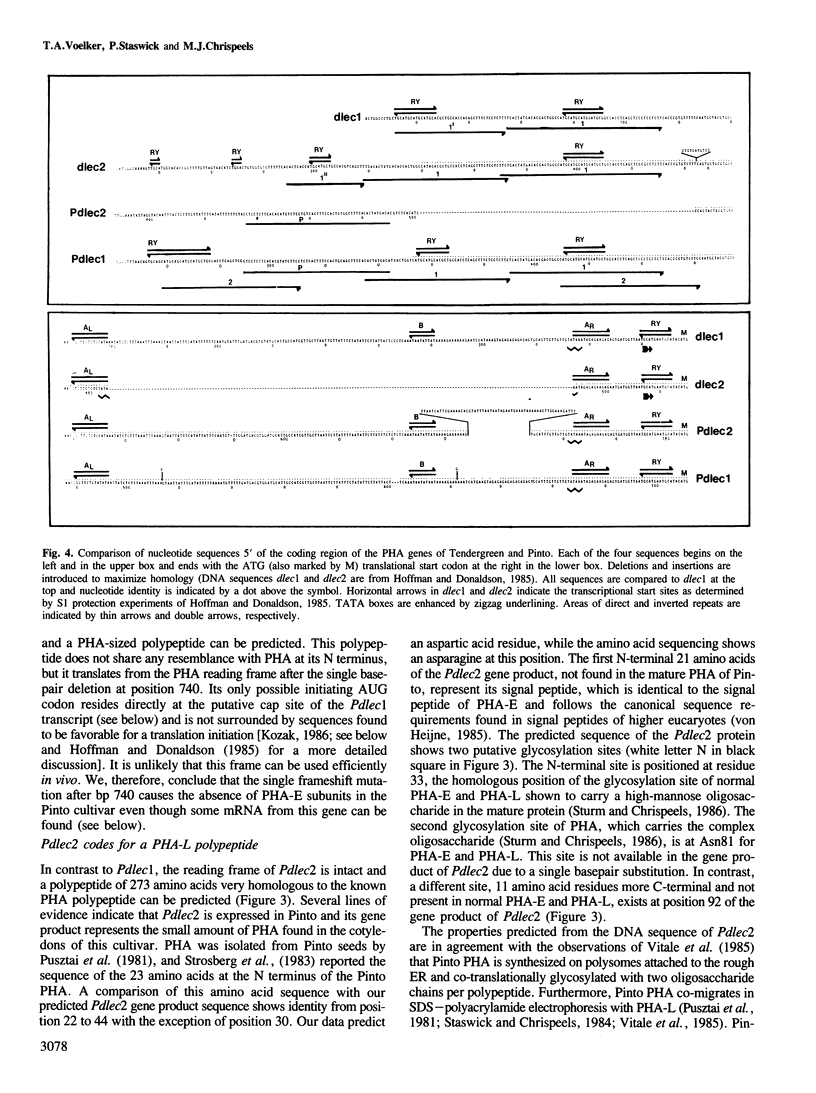
- Hoffman L. M., Donaldson D. D. Characterization of two Phaseolus vulgaris phytohemagglutinin genes closely linked on the chromosome. EMBO J. 1985 Apr;4(4):883–889. doi: 10.1002/j.1460-2075.1985.tb03714.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. Point mutations define a sequence flanking the AUG initiator codon that modulates translation by eukaryotic ribosomes. Cell. 1986 Jan 31;44(2):283–292. doi: 10.1016/0092-8674(86)90762-2. [DOI] [PubMed] [Google Scholar]
- Ladin B. F., Doyle J. J., Beachy R. N. Molecular characterization of a deletion mutation affecting the alpha'-subunit of beta-conglycinin of soybean. J Mol Appl Genet. 1984;2(4):372–380. [PubMed] [Google Scholar]
- Laimins L., Holmgren-König M., Khoury G. Transcriptional "silencer" element in rat repetitive sequences associated with the rat insulin 1 gene locus. Proc Natl Acad Sci U S A. 1986 May;83(10):3151–3155. doi: 10.1073/pnas.83.10.3151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLauchlan J., Gaffney D., Whitton J. L., Clements J. B. The consensus sequence YGTGTTYY located downstream from the AATAAA signal is required for efficient formation of mRNA 3' termini. Nucleic Acids Res. 1985 Feb 25;13(4):1347–1368. doi: 10.1093/nar/13.4.1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J. B., Hsu R., Heinrikson R., Yachnin S. Extensive homology between the subunits of the phytohemagglutinin mitogenic proteins derived from Phaseolus vulgaris. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1388–1391. doi: 10.1073/pnas.72.4.1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proudfoot N. J., Brownlee G. G. 3' non-coding region sequences in eukaryotic messenger RNA. Nature. 1976 Sep 16;263(5574):211–214. doi: 10.1038/263211a0. [DOI] [PubMed] [Google Scholar]
- Rhodes P. R., Vodkin L. O. Highly structured sequence homology between an insertion element and the gene in which it resides. Proc Natl Acad Sci U S A. 1985 Jan;82(2):493–497. doi: 10.1073/pnas.82.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slightom J. L., Sun S. M., Hall T. C. Complete nucleotide sequence of a French bean storage protein gene: Phaseolin. Proc Natl Acad Sci U S A. 1983 Apr;80(7):1897–1901. doi: 10.1073/pnas.80.7.1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staden R. An interactive graphics program for comparing and aligning nucleic acid and amino acid sequences. Nucleic Acids Res. 1982 May 11;10(9):2951–2961. doi: 10.1093/nar/10.9.2951. [DOI] [PMC free article] [PubMed] [Google Scholar]
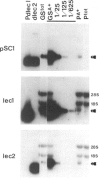
- Staswick P., Chrispeels M. J. Expression of lectin genes during seed development in normal and phytohemagglutinin-deficient cultivars of Phaseolus vulgaris. J Mol Appl Genet. 1984;2(6):525–535. [PubMed] [Google Scholar]
- Strosberg A. D., Lauwereys M., Foriers A. Molecular evolution of legume lectins. Prog Clin Biol Res. 1983;138:7–20. [PubMed] [Google Scholar]
- Sturm A., Chrispeels M. J. The high mannose oligosaccharide of phytohemagglutinin is attached to asparagine 12 and the modified oligosaccharide to asparagine 60. Plant Physiol. 1986 May;81(1):320–322. doi: 10.1104/pp.81.1.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vodkin L. O., Rhodes P. R., Goldberg R. B. cA lectin gene insertion has the structural features of a transposable element. Cell. 1983 Oct;34(3):1023–1031. doi: 10.1016/0092-8674(83)90560-3. [DOI] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- von Heijne G. Signal sequences. The limits of variation. J Mol Biol. 1985 Jul 5;184(1):99–105. doi: 10.1016/0022-2836(85)90046-4. [DOI] [PubMed] [Google Scholar]