Abstract
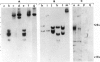
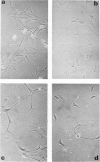
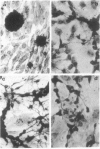
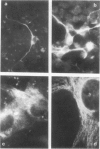
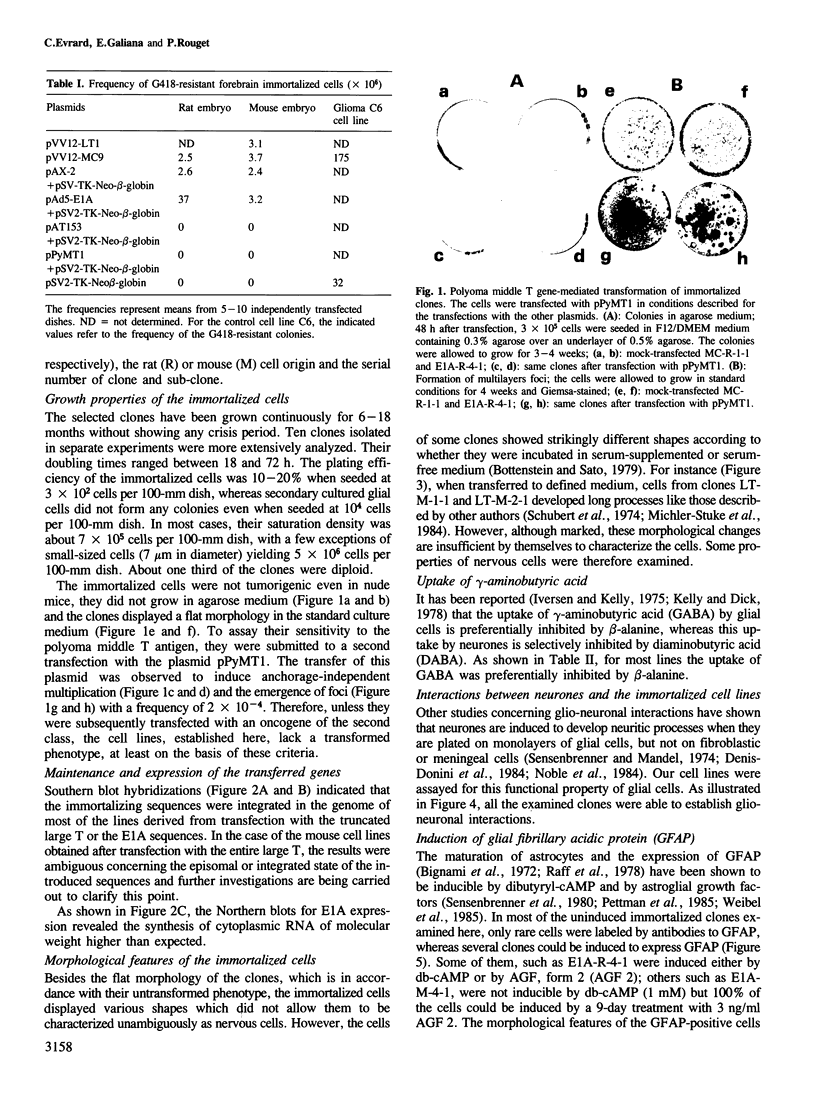
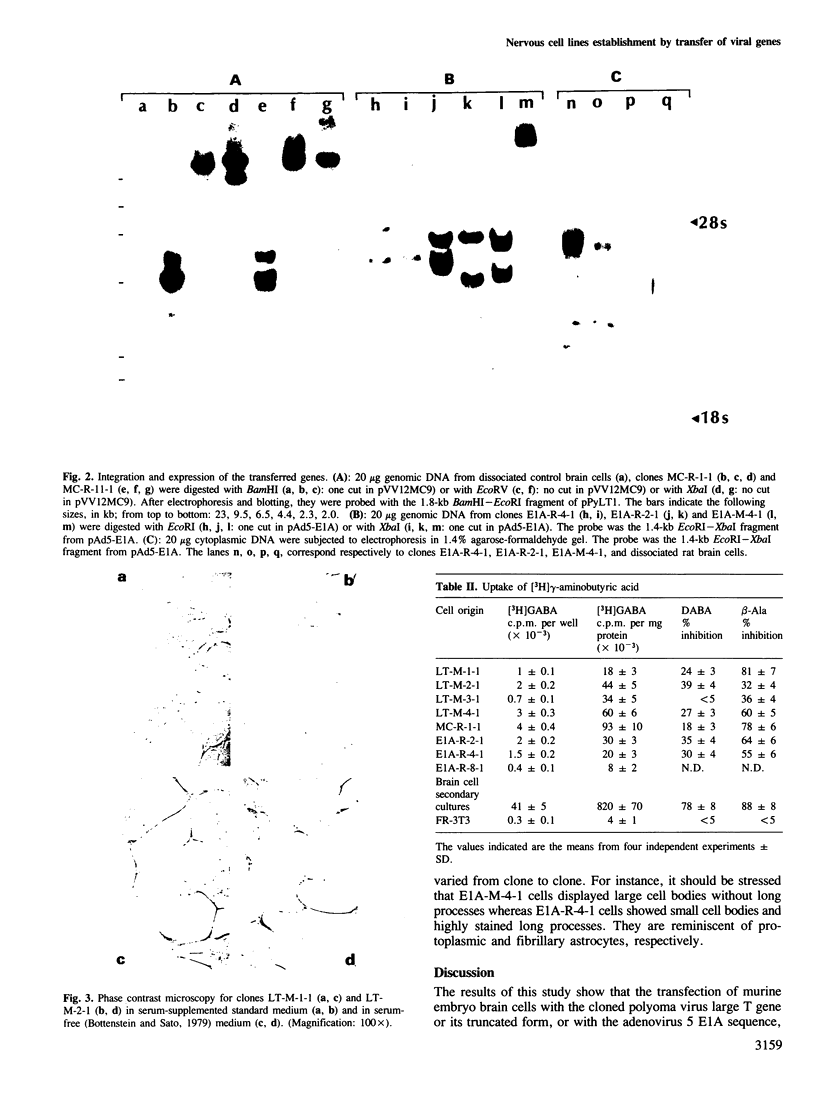
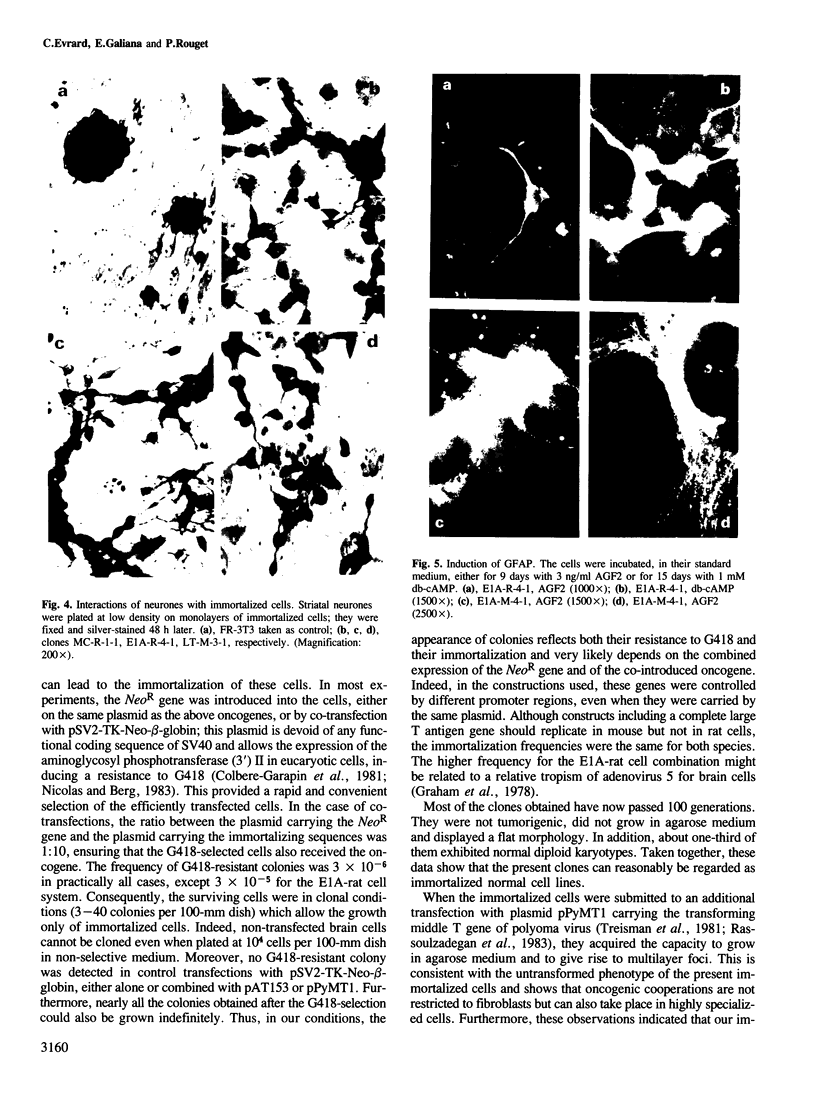
Brain cells from murine embryos were transfected with the polyoma virus large T or the adenovirus 5 EIA gene and, simultaneously, with the phosphotransferase coding NeoR gene. The efficiently transfected cells were selected for their resistance to Geneticin (G418) and their ability to clone at low cell density. Subsequently, most of the selected cells could be sub-cloned and continuously grown for 6-18 months so far. Their doubling time varied between 18 and 72 h. From independent transfections, more than one hundred cell lines were established. They did not exhibit a transformed phenotype, but subsequent transfection with the polyoma middle T gene induced their oncogenic transformation. The maintenance and expression of the transferred genes were verified. Most of the analyzed cell lines retained glial properties. These results suggest that the lines obtained as well as a further extension of this in vitro system should be of interest for the study of nervous cell interactions, differentiation and functions.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alliot F., Pessac B. Astrocytic cell clones derived from established cultures of 8-day postnatal mouse cerebella. Brain Res. 1984 Jul 23;306(1-2):283–291. doi: 10.1016/0006-8993(84)90377-9. [DOI] [PubMed] [Google Scholar]
- Bignami A., Eng L. F., Dahl D., Uyeda C. T. Localization of the glial fibrillary acidic protein in astrocytes by immunofluorescence. Brain Res. 1972 Aug 25;43(2):429–435. doi: 10.1016/0006-8993(72)90398-8. [DOI] [PubMed] [Google Scholar]
- Bottenstein J. E., Sato G. H. Growth of a rat neuroblastoma cell line in serum-free supplemented medium. Proc Natl Acad Sci U S A. 1979 Jan;76(1):514–517. doi: 10.1073/pnas.76.1.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colbère-Garapin F., Horodniceanu F., Kourilsky P., Garapin A. C. A new dominant hybrid selective marker for higher eukaryotic cells. J Mol Biol. 1981 Jul 25;150(1):1–14. doi: 10.1016/0022-2836(81)90321-1. [DOI] [PubMed] [Google Scholar]
- De Vitry F., Camier M., Czernichow P., Benda P., Cohen P., Tixier-Vidal A. Establishment of a clone of mouse hypothalamic neurosecretory cells synthesizing neurophysin and vasopressin. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3575–3579. doi: 10.1073/pnas.71.9.3575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denis-Donini S., Glowinski J., Prochiantz A. Glial heterogeneity may define the three-dimensional shape of mouse mesencephalic dopaminergic neurones. Nature. 1984 Feb 16;307(5952):641–643. doi: 10.1038/307641a0. [DOI] [PubMed] [Google Scholar]
- Edelman G. M. Expression of cell adhesion molecules during embryogenesis and regeneration. Exp Cell Res. 1985 Nov;161(1):1–16. doi: 10.1016/0014-4827(85)90485-9. [DOI] [PubMed] [Google Scholar]
- Favaloro J., Treisman R., Kamen R. Transcription maps of polyoma virus-specific RNA: analysis by two-dimensional nuclease S1 gel mapping. Methods Enzymol. 1980;65(1):718–749. doi: 10.1016/s0076-6879(80)65070-8. [DOI] [PubMed] [Google Scholar]
- Gilardi P., Perricaudet M. The E4 transcriptional unit of Ad2: far upstream sequences are required for its transactivation by E1A. Nucleic Acids Res. 1984 Oct 25;12(20):7877–7888. doi: 10.1093/nar/12.20.7877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham F. L., Harrison T., Williams J. Defective transforming capacity of adenovirus type 5 host-range mutants. Virology. 1978 May 1;86(1):10–21. doi: 10.1016/0042-6822(78)90003-x. [DOI] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Iversen L. L., Kelly J. S. Uptake and metabolism of gamma-aminobutyric acid by neurones and glial cells. Biochem Pharmacol. 1975 May 1;24(9):933–938. doi: 10.1016/0006-2952(75)90422-0. [DOI] [PubMed] [Google Scholar]
- Land H., Parada L. F., Weinberg R. A. Cellular oncogenes and multistep carcinogenesis. Science. 1983 Nov 18;222(4625):771–778. doi: 10.1126/science.6356358. [DOI] [PubMed] [Google Scholar]
- Land H., Parada L. F., Weinberg R. A. Tumorigenic conversion of primary embryo fibroblasts requires at least two cooperating oncogenes. Nature. 1983 Aug 18;304(5927):596–602. doi: 10.1038/304596a0. [DOI] [PubMed] [Google Scholar]
- Loudes C., Faivre-Bauman A., Tixier-Vidal A. Techniques for culture of hypothalamic neurons. Methods Enzymol. 1983;103:313–334. doi: 10.1016/s0076-6879(83)03021-9. [DOI] [PubMed] [Google Scholar]
- Lusky M., Botchan M. Inhibition of SV40 replication in simian cells by specific pBR322 DNA sequences. Nature. 1981 Sep 3;293(5827):79–81. doi: 10.1038/293079a0. [DOI] [PubMed] [Google Scholar]
- Mallat M., Moura Neto V., Gros F., Glowinski J., Prochiantz A. Two simian virus 40 (SV40)-transformed cell lines from the mouse striatum and mesencephalon presenting astrocytic characters. II. Interactions with mesencephalic neurons. Brain Res. 1986 Apr;391(1):23–31. doi: 10.1016/0165-3806(86)90004-0. [DOI] [PubMed] [Google Scholar]
- Noble M., Fok-Seang J., Cohen J. Glia are a unique substrate for the in vitro growth of central nervous system neurons. J Neurosci. 1984 Jul;4(7):1892–1903. doi: 10.1523/JNEUROSCI.04-07-01892.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellegrini S., Dailey L., Basilico C. Amplification and excision of integrated polyoma DNA sequences require a functional origin of replication. Cell. 1984 Apr;36(4):943–949. doi: 10.1016/0092-8674(84)90044-8. [DOI] [PubMed] [Google Scholar]
- Pessac B., Girard A., Romey G., Crisanti P., Lorinet A. M., Calothy G. A neuronal clone derived from a Rous sarcoma virus-transformed quail embryo neuroretina established culture. Nature. 1983 Apr 14;302(5909):616–618. doi: 10.1038/302616a0. [DOI] [PubMed] [Google Scholar]
- Raff M. C., Mirsky R., Fields K. L., Lisak R. P., Dorfman S. H., Silberberg D. H., Gregson N. A., Leibowitz S., Kennedy M. C. Galactocerebroside is a specific cell-surface antigenic marker for oligodendrocytes in culture. Nature. 1978 Aug 24;274(5673):813–816. [PubMed] [Google Scholar]
- Rassoulzadegan M., Cowie A., Carr A., Glaichenhaus N., Kamen R., Cuzin F. The roles of individual polyoma virus early proteins in oncogenic transformation. Nature. 1982 Dec 23;300(5894):713–718. doi: 10.1038/300713a0. [DOI] [PubMed] [Google Scholar]
- Rassoulzadegan M., Naghashfar Z., Cowie A., Carr A., Grisoni M., Kamen R., Cuzin F. Expression of the large T protein of polyoma virus promotes the establishment in culture of "normal" rodent fibroblast cell lines. Proc Natl Acad Sci U S A. 1983 Jul;80(14):4354–4358. doi: 10.1073/pnas.80.14.4354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Ruley H. E. Adenovirus early region 1A enables viral and cellular transforming genes to transform primary cells in culture. Nature. 1983 Aug 18;304(5927):602–606. doi: 10.1038/304602a0. [DOI] [PubMed] [Google Scholar]
- Schubert D., Heinemann S., Carlisle W., Tarikas H., Kimes B., Patrick J., Steinbach J. H., Culp W., Brandt B. L. Clonal cell lines from the rat central nervous system. Nature. 1974 May 17;249(454):224–227. doi: 10.1038/249224a0. [DOI] [PubMed] [Google Scholar]
- Sensenbrenner M., Devilliers G., Bock E., Porte A. Biochemical and ultrastructural studies of cultured rat astroglial cells: effect of brain extract and dibutyryl cyclic AMP on glial fibrillary acidic protein and glial filaments. Differentiation. 1980;17(1):51–61. doi: 10.1111/j.1432-0436.1980.tb01081.x. [DOI] [PubMed] [Google Scholar]
- Sensenbrenner M., Mandel P. Behaviour of neuroblasts in the presence of glial cells, fibroblasts and meningeal cells in culture. Exp Cell Res. 1974 Jul;87(1):159–167. doi: 10.1016/0014-4827(74)90538-2. [DOI] [PubMed] [Google Scholar]
- Sobue G., Pleasure D. Astroglial proliferation and phenotype are modulated by neuronal plasma membrane. Brain Res. 1984 Dec 17;324(1):175–179. doi: 10.1016/0006-8993(84)90639-5. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Sylla B. S., Huberdeau D., Bourgaux-Ramoisy D., Bourgaux P. Site-specific excision of integrated polyoma DNA. Cell. 1984 Jun;37(2):661–667. doi: 10.1016/0092-8674(84)90398-2. [DOI] [PubMed] [Google Scholar]
- Treisman R., Novak U., Favaloro J., Kamen R. Transformation of rat cells by an altered polyoma virus genome expressing only the middle-T protein. Nature. 1981 Aug 13;292(5824):595–600. doi: 10.1038/292595a0. [DOI] [PubMed] [Google Scholar]
- Wahl G. M., Stern M., Stark G. R. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. doi: 10.1073/pnas.76.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Z. Y., Veldman G. M., Cowie A., Carr A., Schaffhausen B., Kamen R. Construction and functional characterization of polyomavirus genomes that separately encode the three early proteins. J Virol. 1984 Jul;51(1):170–180. doi: 10.1128/jvi.51.1.170-180.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Elsen P., de Pater S., Houweling A., van der Veer J., van der Eb A. The relationship between region E1a and E1b of human adenoviruses in cell transformation. Gene. 1982 May;18(2):175–185. doi: 10.1016/0378-1119(82)90115-9. [DOI] [PubMed] [Google Scholar]