Abstract
Widely distributed in nature, sulfated glycan epitopes play important roles in diverse pathophysiological processes. However, due to their structural complexity, the preparation of glycan epitopes with structurally defined sulfation patterns is challenging, which significantly hampers the detailed elucidation of their biological functions at the molecular level. Here, we introduce a strategy for site-specific chemical sulfation of glycan epitopes, leveraging enzymatic sialylation and desialylation processes to precisely control the regio-specificity of sulfation of disaccharide or trisaccharide glycan backbones. Using this method, a sulfated glycan library covering the most common sialylated glycan epitopes was prepared in high yield and efficiency. By screening a microarray prepared with this glycan library, we systematically probed their binding specificity with human Siglecs (sialic acid-binding immunoglobulin-type lectins), many of which function as glyco-immune checkpoints to suppress immune system activation. Our investigation revealed that sulfation and sialylation patterns serve as important determinants of Siglec binding affinity and specificity. Thus, these findings offer new insights for the development of research tools and potential therapeutic agents targeting glyco-immune checkpoints by modulating the Siglec signaling pathway.
Introduction
Glycan sulfation is a ubiquitous and crucial post-translational modification that widely occurs in glycoproteins, glycolipids, and proteoglycans.1,2 Sulfation imparts negative charges to the modified glycans, giving them specificity for interaction with numerous human and microbial glycan binding proteins to regulate diverse biological processes.3−7 On the other hand, abnormal glycan sulfation has been linked to human diseases, such as cancers and osteoarthritis.8−12 Sialic acid-binding immunoglobulin-type lectins (Siglecs) are a family of transmembrane receptors with restricted expression on immune cells that regulate the immune cell activities through the engagement of sialoglycans that are often sulfated.13−15 In humans, 15 Siglec receptors have been identified.16,17 According to their intracellular signaling domains, Siglec receptors can be classified into three subgroups, including nonsignaling receptors (Siglec-1 and -4), activating receptors (Siglec-14, -15, and -16), and inhibitory receptors (all other Siglecs).17,18 In the immunological synapse, inhibitory Siglecs engage with sialylated glycan epitopes, initiating inhibitory signaling that suppresses immune cell activation in a manner similar to the interaction observed between PD-1 and PD-L1.19,20 Thus, such Siglecs are designated as glyco-immune checkpoints. A growing body of evidence has shown that sulfation occurring on the primary hydroxyls of terminal monosaccharide residue, for example, Gal, GlcNAc, and GalNAc, of sialoglycans is involved in modulating the binding affinity of many Siglecs.21−23 Sulfated glycan epitopes, which are defined as structures containing disaccharide or trisaccharide backbone, can be explored as ligands to deliver diagnostic or therapeutic agents to diverse immune cells that express specific Siglecs.21,24−30 For these applications, there is an urgent need to comprehend the biological mechanisms underlying subtle differences in sulfation patterns governing the Siglec binding specificities. However, sulfated glycans are not readily accessible due to their structural heterogeneity and complexity.1 Therefore, the detailed roles that sulfated glycans played in living cells are still largely unexplored.
Given the significance of sulfated glycan epitopes in both fundamental research and pharmaceutical chemistry, many chemical approaches have been explored for their preparation.31−34 Total chemical synthesis of sulfated glycans needs complex protection–deprotection manipulations for the site-specific introduction of the sulfate group. Moreover, the poor solubility of sulfated glycans in organic solvents and the instability of the sulfate group in acid conditions add extra difficulty to the already challenging synthesis of the sulfated glycan backbones.35 Alternatively, Chen and co-workers reported a chemoenzymatic approach to prepare sulfated sialyl Lewis x (SLeX) glycan epitope.36 By this method, the sulfated glycan backbones, which need to be prepared by total chemical synthesis, were modified by sialyltransferases to produce SLeX antigens. Similarly, Cao and co-workers prepared sulfated O-Mannose glycan epitopes.37 Recently, Wang and co-workers synthesized sulfated antibody glycoforms (sulfated Galβ1,4GlcNAc backbone) using human GlcNAc-6-O-sulfotransferase and human β1,4 galactosyltransferase.38 Later, Boons and co-workers synthesized complex sulfated keratan glycans and N-glycans (sulfated Galβ1,4GlcNAc backbone) by using the same enzymes.39,40 Now, there is still a lack of a general method for the rapid construction of a number of structure-defined sulfated glycans containing other glycan backbones.
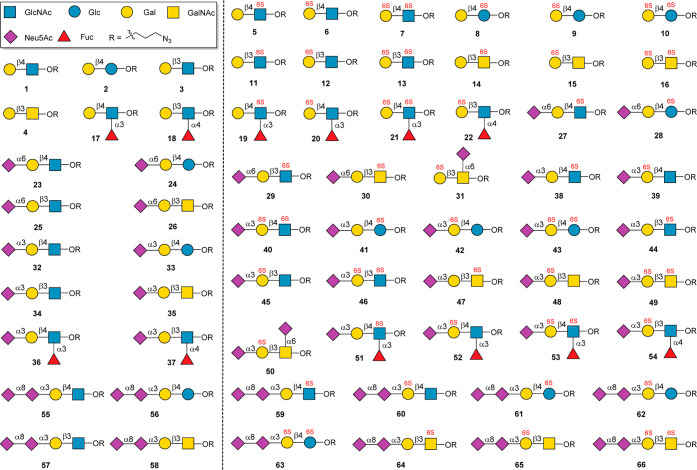
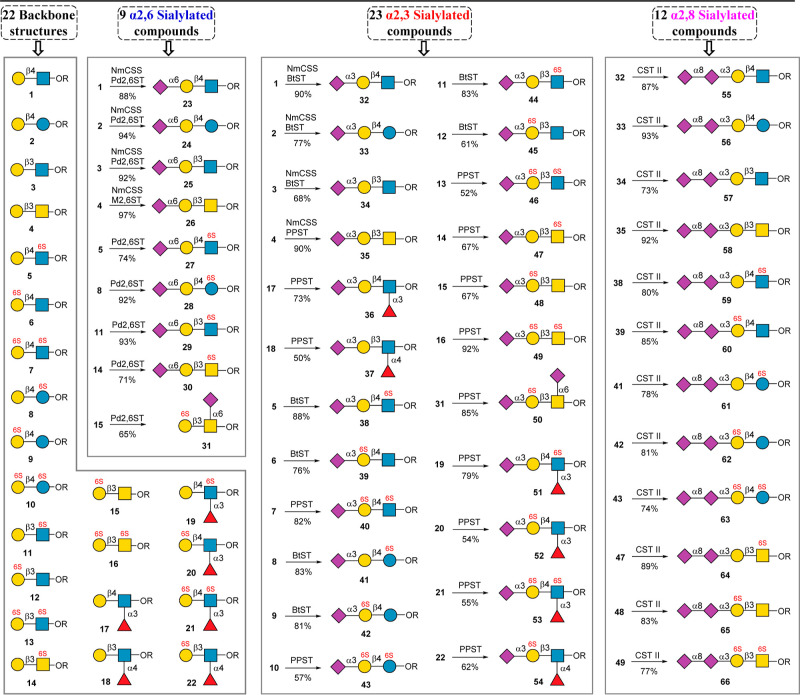
Here, we describe an enzyme-sialylation-controlled strategy for the site-specific chemical sulfation of glycan epitopes bearing disaccharide or trisaccharide backbones. Taking advantage of enzyme-catalyzed sialylation as a protecting group, which could protect the highly reactive C-6 or C-3 hydroxyl group (OH) of Gal residue, the sulfate group could be introduced into disaccharide or trisaccharide backbones specifically in mild chemical reaction conditions. The protective sialic acid residue can be removed later by neuraminidase, resulting in general glycan backbones containing mono- or disulfate groups. The further extension of these sulfated glycan backbones by several glycosyltransferases produced a 66-membered glycan library covering the most common sialylated glycan epitopes (Figure 1). The constructed well-defined glycan microarray was subsequently used to probe the binding specificities of human Siglecs, revealing many new findings about the specificity of Siglecs for sulfated glycans.
Figure 1.
Structures of 66-membered glycan library.
Results and Discussion
Although human glycan structures are complex, the backbones are much simple. Galβ1,4GlcNAc (Type II LacNAc), Galβ1,4Glc (Lactose), Galβ1,3GlcNAc (Type I LacNAc), and Galβ1,3GalNAc are the four basic backbones of N-glycans, O-glycans, and glycolipids. Glycan sulfation is mediated by sulfotransferases using 3′-phosphoadenosine-5′-phosphosulfate (PAPS) as the sulfate donor in living cells. However, it is challenging to scale up the synthesis of PAPS in laboratories since PAPS is unstable in solution and commercial PAPS is prohibitively expensive for large-scale glycan sulfation. Moreover, human sulfotransferases need to be expressed by using an eukaryotic expression system, and many sulfotransferases suffer from low expression.41 In addition, sulfotransferases are often associated with low activities, and a mass of enzymes need to be added in the reaction system.39,40 For these reasons, we sought to develop a chemical approach that would enable the incorporation of sulfate and Neu5Ac site, specifically onto the most common glycan epitopes found in N- and O-linked glycans, i.e., Galβ1,4GlcNAc, Galβ1,4Glc, Galβ1,3GlcNAc, and Galβ1,3GalNAc.42
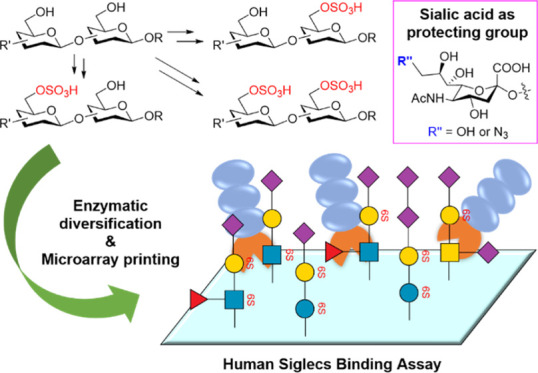
The well-known chemical approach to install the sulfate group relies on the use of SO3 complexes, such as SO3/Py, which primarily installs sulfate onto the C-6 OH of sugar residues43,44 but cannot differentiate C-6 OH located on different sugar residues. Sometimes, SO3/Py also introduces sulfate onto C-3 OH.44 To achieve site-specific sulfation, we took advantage of α2,3 sialyltransferase- or α2,6 sialyltransferase-mediated sialylation to introduce a sialic acid as a temporary “protecting group”, which specifically blocks the C-6 OH or C-3 OH of the terminal Gal residue, respectively. Chemical sulfation can then be conducted to introduce a sulfate group to the C-6 OH of a desired monosaccharide residue. Finally, removal of the protecting group by neuraminidase produced the sulfated glycan backbones (Figures 2 and 4–6), which can be further elaborated by glycosyltransferases to give structure-defined sulfated glycans. Notably, in 2019, Cao and co-workers pioneered the use of sialic acid as a temporary “protecting group” for site-specific enzymatic introduction of fucose residue in the LacNAc backbone.7
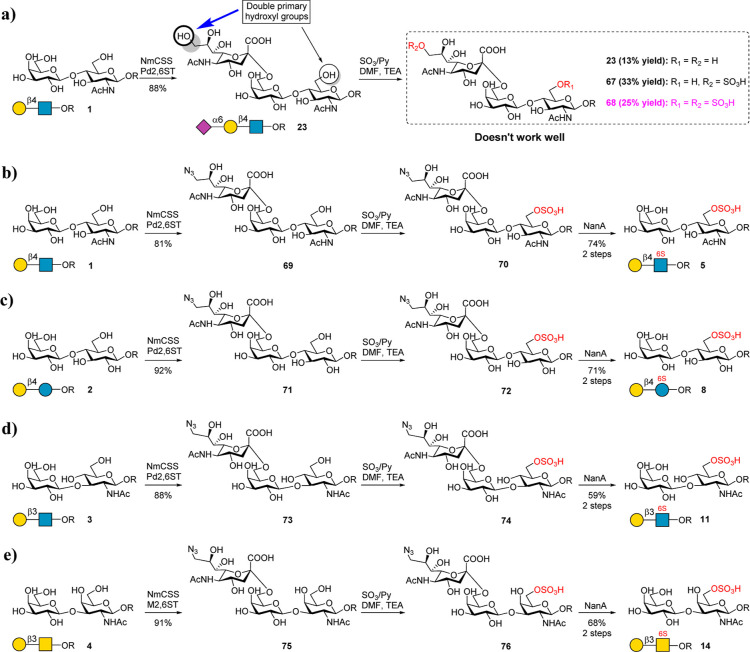
Figure 2.
Chemoenzymatic preparation of disaccharide backbones 5, 8, 11, and 14. (a) Use of Neu5Ac as a protecting group to prepare 5. The condition of chemical sulfation is SO3/Py (12 equiv), DMF/TEA (V/V = 7:3), 0 °C–r.t., and 2 h. (b–e) Use of 9-N3–Neu5Ac as the protecting group to prepare 5, 8, 11, and 14. The condition of chemical sulfation is SO3/Py (8 equiv), DMF/TEA (V/V = 9:1), 0 °C–r.t., and 1–2 h. The condition of 9-N3–Neu5Ac hydrolysis is NaOAc buffer (pH 6.5), NanA, and 4 h.
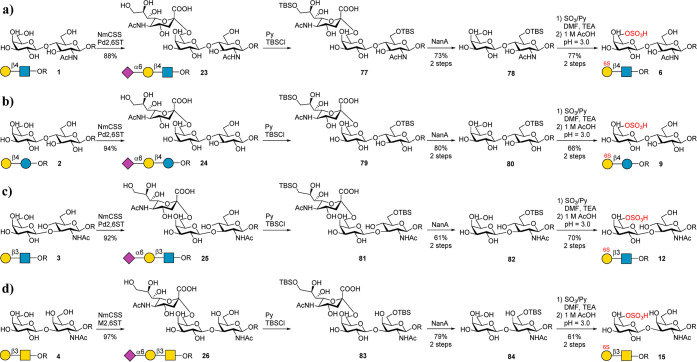
Figure 4.
Chemoenzymatic preparation of disaccharide backbones 6, 9, 12, and 15. The condition of chemical sulfation is SO3/Py (4 equiv), DMF/TEA (V/V = 1:1), 0 °C–40 °C, and 3–4 h. Deprotection of TBS is performed by adjusting pH to 3.0 using 1 M AcOH(aq).
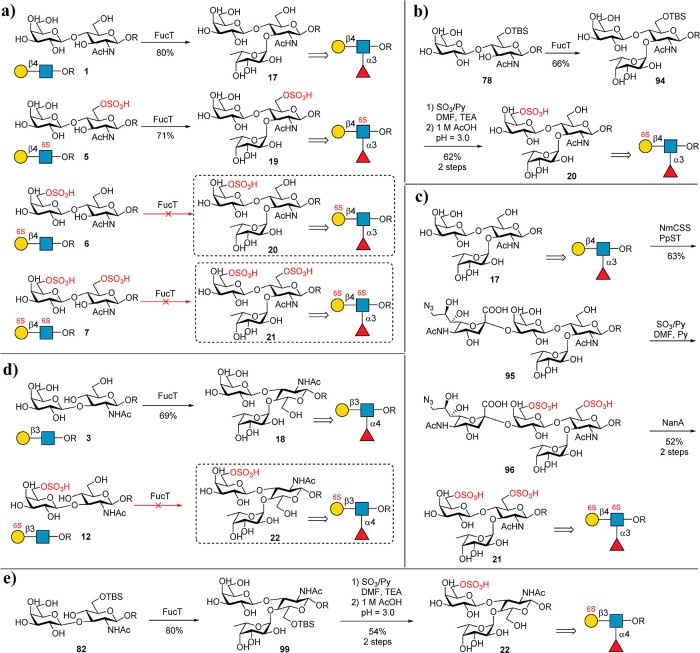
Figure 6.
Chemoenzymatic preparation of fucosylated backbones 17, 18, 19, 20, 21 and 22. The condition of chemical sulfation is SO3/Py (4 equiv), DMF/TEA (V/V = 1:1), 0–40 °C, and 3–4 h for (b,e) and SO3/Py (15 equiv), DMF/Py (V/V = 1:1), 0 °C, and 1–2 h for (c).
To test the proposed strategy, we first synthesized 5 from 1. A linker containing an azido group (azido propane, ProN3) was designed for microarray printing. Initially, we tried to use Neu5Ac as a protecting group to block the C-6 OH of Gal for site-selective chemical sulfation of the C-6 OH of GlcNAc (Figure 2a). To this end, 23 was prepared from 1 by a one-pot reaction using CMP-Sialic acid synthetase from Neisseria meningitidis (NmCSS)45 and α2,6 sialyltransferase from Photobacterium damselae (Pd2,6ST).46 The enzymes were prepared as reported previously.47,48 Subsequently, 23 was treated with SO3/Py in a mixed solvent of DMF and TEA at room temperature for 2 h (Figure 2a). However, the reaction produced a mixture, and three components were isolated, including starting material 23 (13% yield), 67 (9S-Neu5Acα2,6Galβ1,4GlcNAcProN3; 33% yield), and 68 (9S-Neu5Acα2,6Galβ1,4(6S)GlcNAcProN3; 25% yield) (Figure S1). This result indicated that the C-9 OH of Neu5Ac possesses a higher reactivity than the C-6 OH of GlcNAc in 23. Although 68 can also be hydrolyzed by neuraminidase from Streptococcus pneumoniae (NanA, α2,3/6/8 sialidase)49 to give the target product 5, the total yield was very low.
To address this issue, we chose 9-N3–Neu5Ac as a temporary “protecting group” due to its lack of a primary OH at the C-9 position (Figure 2b). 9-N3–Neu5Ac was prepared from Neu5Ac through three facile chemical steps.50,51 It was then used to prepare 69 by using Pd2,6ST and NmCSS in a one-pot reaction. Next, 69 was treated with SO3/Py in a mixed solvent of DMF and TEA to produce target intermediate 70 as a primary product (Figure 2b). Further treatment of 70 with NanA successfully gave the target backbone 5 with a high yield (74%, two steps from 69). Notably, although three chemical steps are needed to prepare 9-N3–Neu5Ac, 9-N3–Neu5Ac can be recycled after hydrolysis and purification by a size-exclusion column for further enzymatic reaction. Similarly, sulfated disaccharides 8, 11, and 14 were also synthesized successfully in high yields (Figure 2c–e). All products were confirmed by 1D and 2D NMR and HRMS analyses (see the Supporting Information). The sulfate group position of the products can be clearly distinguished by comparison with the 1H NMR spectrum of nonsulfated structures (Figure 3). Indeed, it was reported that several β1,4 galactosyltransferases can recognize 6S-GlcNAc and convert 6S-GlcNAc to Galβ1,4(6S)GlcNAc using UDP-Gal as a donor.38,43 However, other backbones Galβ1,4Glc, Galβ1,3GlcNAc, and Galβ1,3GalNAc cannot be synthesized enzymatically due to the lack of suitable galactosyltransferases. Although chemical synthesis of 5, 8, 11, and 14 can also be achieved by the strategy design,44,52−54 the processes are tedious and suffer from low yields. For example, the chemical synthesis of 5 needs more than 12 steps with a total yield of less than 8.5%.52 Moreover, each backbone needs a different synthetic route. Thus, we present here a general strategy for the efficient preparation of 5, 8, 11, and 14.
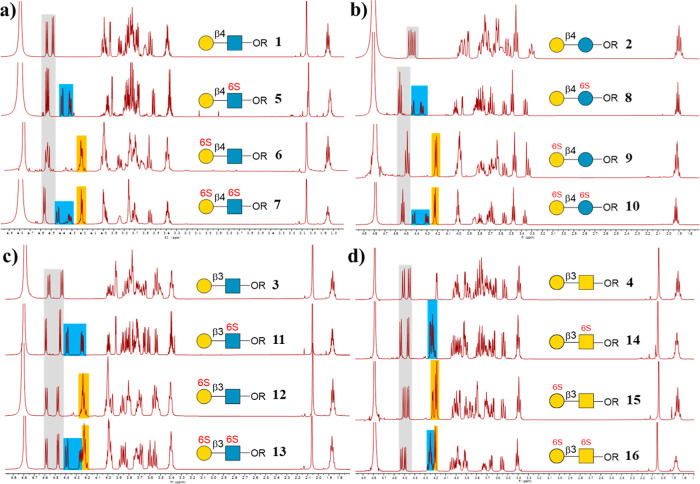
Figure 3.
Presentation of the 1H NMR spectrum of nonsulfated and sulfated glycan backbones. The gray squares indicate the anomeric protons. The blue squares indicated the C-6 position protons of GlcNAc, Glc, or GalNAc. The yellow squares indicate the C-6 position protons of Gal.
Subsequently, we attempted to synthesize sulfated disaccharides 6, 9, 12, and 15, which contain a sulfate group at the C-6 OH of Gal (Figure 4). The challenge is to selectively protect the C-6 OH of GlcNAc to prevent its sulfation. The tert-butyldimethylsilyl (TBS) was chosen as the protecting group. 23 was treated with tert-butyldimethylsilyl chloride (TBSCl) in pyridine (Figure 4a), affording absolute selectivity to produce compound 77 as the major product, which, upon hydrolysis by NanA, gave the intermediate 78. Then, 78 was treated with SO3/Py and TEA in DMF at 40 °C for 2 h to chemically sulfate the C-6 OH of Gal. It is worth mentioning that the C-3 OH of Gal was also sulfated to some extent. This byproduct can be avoided by controlling the reaction time and temperature. The evaporated reaction crude was then dissolved in water, and the pH was adjusted to 3.0 with 1 M aqueous AcOH to deprotect the TBS group, giving the target product 6 in 77% yield with respect to 78. In contrast, the chemical synthesis of 6 needs 15 steps (13% yield).36 Similarly, the backbones (9, 12, and 15) were also prepared successfully in excellent yields (Figure 4b–d). All products were confirmed by 1D and 2D NMR and HRMS analysis (see the Supporting Information). The sulfate group position of the products can be clearly distinguished by comparison with the 1H NMR spectrum of nonsulfated structures (Figure 3).
As the next step, we synthesized disulfated backbones 7, 10, 13, and 16. Initially, we tried to prepare 7 from 1 by treating 1 directly with SO3/Py and TEA in DMF as described above. However, the reaction was rather messy, and NMR analysis indicated that the main product contained only one sulfate group (data not shown). Therefore, 1 was treated in a more intensive sulfation reaction condition (Figure 5a). However, the main product was a trisulfated glycan 85 containing an additional sulfate group at C-3 OH of Gal (Figures 5b and S2). This result again proved that the C-3 OH of the Gal residue has a relatively higher reactivity under intensive sulfation conditions. To address this issue, we designed a strategy as shown in Figure 5c–f to prepare disulfated backbones 7, 10, 13, and 16. In this approach, the C-3 OH of the terminal Gal was protected by an α2,3-linked 9-N3–Neu5Ac by using α2,3 sialyltransferase. To synthesize 7 from 1 (Figure 5c), 1 was first converted to 86 by using Bibersteinia trehalosi α2,3 sialyltransferase (BtST).55,56 Comparing with the well-known α2,3-sialyltransferase from Pasteurella multocida (PmST1), BtST processes low sialidase activity and donor hydrolysis activity, while no α2,6-sialyltransferase and trans-sialidase activity were observed. In addition, the use of 9-N3–Neu5Ac as a “protecting group” could avoid unwanted byproducts. 86 was further treated with SO3/Py at 0 °C for 1 h. As expected, disulfated glycan 87 was produced as the main product. 87 was then treated with NanA to hydrolyze 9-N3–Neu5Ac to give the target product 7 in a total yield of 50% for 3 steps. Meanwhile, total chemical synthesis of 7 needs over 16 steps.36 Similarly, backbones 10, 13, and 16 were also successfully prepared using the described strategy (Figure 5d–f). All products were confirmed by 1D and 2D NMR and HRMS analysis (see the Supporting Information). As mentioned above, the sulfate group position of products can be clearly distinguished by comparison with the 1H NMR spectrum of nonsulfated structures (Figure 3).
Figure 5.
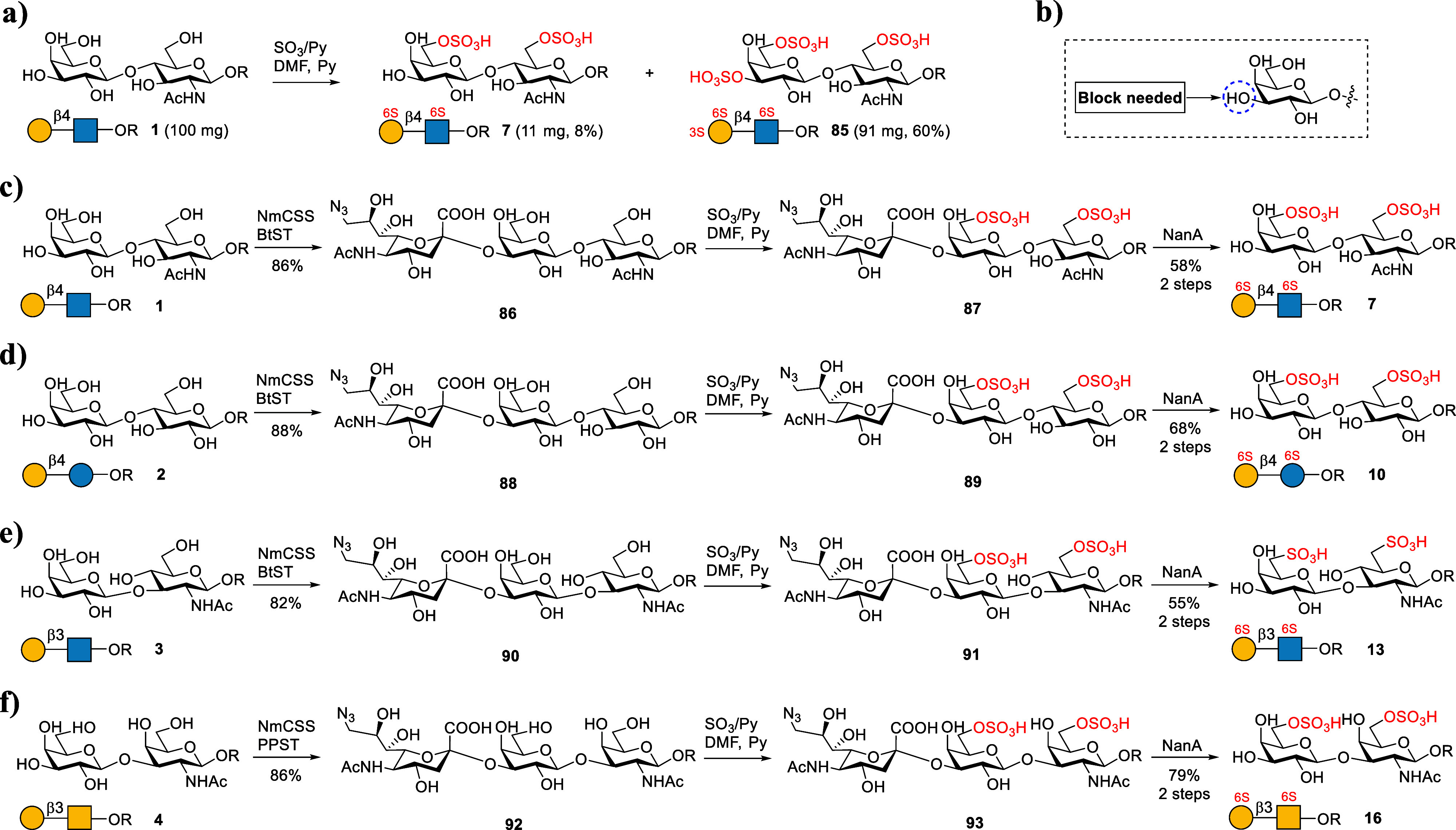
Chemoenzymatic preparation of backbones 7, 10, 13, and 16. The condition of chemical sulfation is SO3/Py (15 equiv), DMF/Py (V/V = 1:1), 0 °C, and 1–2 h.
Backbones Galβ1,4GlcNAc and Galβ1,3GlcNAc are frequently fucosylated in natural glycans.57 Therefore, we next sought to prepare a sulfated version of fucosylated glycan backbones 17 to 22. Initially, we tried to synthesize 17, 19, 20, and 21 from 1, 5, 6, and 7 (Figure 6a) using Helicobacter pylori α1,3/4 fucosyltransferase (FucT).58 However, only 1 and 5 can be accepted by FucT to give glycans 17 (also known as the sulfated Lewis X (LeX) antigen) and 19 in excellent yields, while 6 and 7 cannot be recognized by FucT (Figure 6a). It is possible that the sulfate group at the C-6 OH of Gal inhibits the fucosylation by FucT, whereas FucT accepts the modification at C-6 OH of GlcNAc. Inspired by this assumption, we investigated the activity of FucT toward 78 (Figure 6b). We found that FucT indeed accepts 78 as a substrate to produce 94, which was then selectively sulfated, as described above. After the removal of the TBS group, the target glycan backbone 20 was formed in 41% total yield with respect to 78. Compared to the total chemical synthesis of 20,36 which requires complicated protection/deprotection manipulations, the strategy described here is more efficient. As FucTs did not accept substrates containing modification at the C-6 OH of the Gal residue, we designed a new synthetic route to prepare backbone 21 from 17 (Figure 6c). However, we found that BtST could not accept 17 as a substrate. The use of PmST1M144D results in a high ratio of byproducts containing α2,6-linked Neu5Ac (data not shown). This is probably because PmST1 has weak α2,3-sialidase activity, which leads to the cleavage of α2,3-sialyl linkages slowly but leaves α2,6-sialyl linkages intact. Thus, we cloned and screened many other α2,3 sialyltransferases. Finally, we found an α2,3 sialyltransferases from Photobacterium phosphoreum (PPST),56,59 could recognize 17 efficiently, while no byproduct containing α2,6-linked Neu5Ac was observed. More importantly, it also accepted CMP-9-N3–Neu5Ac as a donor. Using PPST, intermediate 95 was successfully obtained from 17 in a high yield (67%). 95 was then treated with a chemical sulfation reaction to introduce two sulfate groups to produce intermediate 96. The hydrolysis of 9-N3–Neu5Ac by NanA produced the target product 21 in 52% yield with respect to 17. Similarly, 18 was prepared successfully from 3 in a 69% yield. 22 was prepared from 82 in 54% yield (Figure 6e) as the strategy for the preparation of 20. Meanwhile, the preparation of 97 and 98 from 11 and 13 is unsuccessful due to the tested α1,3/4 fucosyltransferases from H. pylori (NCTC 11369 and UA948) that could not recognize both substrates (Figure S3).
With backbones 1 to 22 in hand, we next conducted enzymatic sialylation reactions to construct the glycan library shown in Figure 1. In humans, Neu5Ac is typically linked to the Gal residue by an α2,3 or α2,6 linkage, while Neu5Ac can be linked to another Neu5Ac by an α2,8 linkage. The sialylation reaction toward the nonsulfated sialoglycans was performed in a one-pot reaction manner containing CTP, Neu5Ac, and NmCSS, which could avoid the use of pure CMP-Neu5Ac as a glycosylation donor. Meanwhile, the sialylation reaction toward the sulfated sialoglycans was performed using CMP-Neu5Ac as the glycosylation donor for the convenience of reaction detection by TLC. 23 to 31, which contain α2,6-linked Neu5Ac, were efficiently synthesized as described above using Pd2,6ST (26 was prepared using Pd2,6ST mutant(M2,6ST)). α2,3 Sialylation was performed by using BtST or PPST to produce 23 structures (Figure 7). 35 to 37, 40 to 43, and 46 to 54 were prepared by using PPST because PPST gives higher yields or many backbones such as 7, 10, 13, 16, and 17 to 22 cannot be accepted by BtST. Other structures shown in Figure 7 were prepared by using BtST. We next chose 12 important α2,3 sialoglycans for further extension by α2,3/8 sialyltransferase from Campylobacter jejuni (CST II).60 The selected glycans include nonsulfated glycans, monosulfated glycans, and disulfated glycans, and all selected substrates can be well accepted by CST II to produce 12 structures (55 to 66) containing both α2,3- and α2,8-linked Neu5Ac. All of the above synthesized compounds were purified by using size-exclusion and ion-exchange columns. The obtained glycans, including 20 nonsulfated glycans and 46 sulfated glycans, were confirmed by 1D and 2D NMR and HRMS analysis (see the Supporting Information).
Figure 7.
Preparation of sialoglycans from 22 backbones.
With the 66 synthetic glycans in hand, we further treated them with Pd(OH)2/C under H2 pressure to reduce the linker of ProN3 to ProNH2. The reduced glycans were then printed onto amine-reactive N-hydroxysuccinimide (NHS)-activated glass slides to construct a glycan microarray. The array was probed with five well-known plant lectins including Maackia amurensis Lectin I (MAL I, binds terminal Neu5Acα2,3Galβ1,4GlcNAc),61,62Sambucus nigra Lectin (SNA, binds Neu5Acα2,6Galβ1,4GlcNAc),63,64Erythrina cristagalli Lectin (ECL, binds Galβ1,4GlcNAc),65Arachis hypogaea Lectin (PNA, binds Galβ1,3GalNAc),66,67 and Aleuria aurantia Lectin (AAL, binds l-fucose residue).68,69 Microarray screening provided the expected binding specificities to these plant lectins (Figure S4), confirming the feasibility of the constructed glycan microarray for use in protein-binding studies.
We next investigated Siglecs binding with a constructed glycan microarray. Among 15 Siglecs in humans, Siglec-12 carries a mutation (R122C) and loses the ability to bind sialoglycans.70 Siglec-5 and -14 and Siglec-11 and -16 are paired receptors that share the same ligand-binding preferences.71−73 Therefore, we chose to study the binding preference of recombinant Siglec-1-Fc to Siglec-11-Fc and Siglec-15-Fc with the constructed microarray, in which the binding avidity was assessed using fluorescently labeled antihuman Fc followed by scanning with a microarray reader (647 nm).
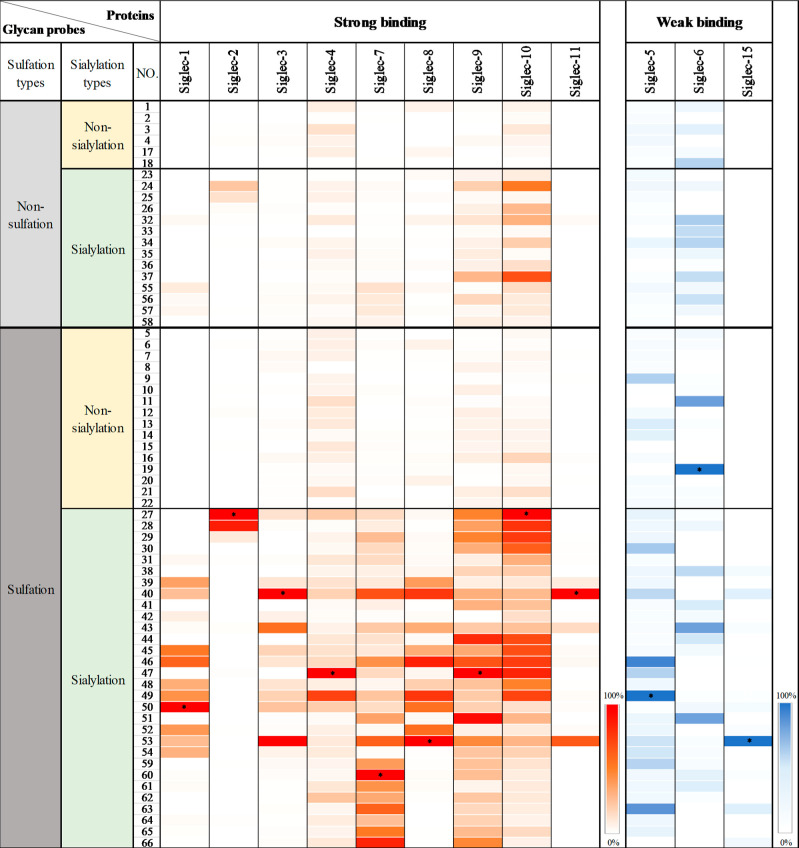
As summarized in Figure 8, glycan sulfation, in general, enhanced the binding ability of ligands to Siglecs, which is consistent with the previous reports that Siglecs have a preference for sulfated glycan epitopes.13,14,17 For example, in line with what was reported by Crocker et al., 27, which contains both Neu5Ac α2,6 linked to Type II LacNAc (Galβ1,4GlcNAc) and a sulfate group at the 6-OH position of the inner GlcNAc but not the unsulfated glycan 23, exhibited strong binding to Siglec-2, a B-cell-associated inhibitory receptor.15 In accordance with Paulson’s observation, a strong binding of 28 to Siglec-2 was also observed. In addition, the glycan-binding patterns for Siglec-3, -7, -8, and -9 revealed by our screening were also consistent with what has been reported previously.6,22,23 Importantly, we found that the glycan 60, harboring both α2,8 sialylation and Gal-6-O-sulfation, presented an additive effect on Siglec-7 binding compared to 39 and 55.
Figure 8.
Binding profiles of human Siglecs with the 66-membered glycan microarray. *The strongest binding signal of the tested Siglec is defined as 100%. See the histogram charts of binding results in the Supporting Information (Figure S5).
Significantly, our screening also revealed some novel patterns of Siglec-binding specificity. For example, a previous study showed that Siglec-3 and Siglec-8 share a similar ligand-binding preference.39 However, we found that the disulfated glycans 46 and 49 with the β1,3 Gal backbone exhibited a strong binding to Siglec-8 but a weak binding to Siglec-3, suggesting that not only sialylation and sulfation patterns but also the backbone structure of the ligand may influence recognition by Siglecs. Similarly, the ligand backbone structures were also found to influence binding to Siglec-9. Specifically, 44 and 47 displayed binding affinities to Siglec-9 stronger than those of 38 and 41, suggesting that Siglec-9 prefers sulfated α2,3-linked sialoglycans with Type I LacNAc or Galβ1,3GalNAc backbones. In addition, our screening data showed that disulfated 3′SLN (40) and SLex (53) bound strongly to both Siglec-3 and -8, indicating that the presence of α1,3 fucose on Type II LacNAc had no effect on the binding. Similarly, Siglec-11 also showed the highly specific binding to disulfated 3′SLN (40) and SLex (53). On the other hand, the interaction between Siglec-8 and 45 was abrogated when α1,4 fucose was conjugated to Type I LacNAc (54).
Furthermore, our binding experiments revealed how site-specific sulfation patterns regulate ligand binding to Siglec-1,74,75 Siglec-4,22,76 and -10,77−79 whose ligand specificities were largely unidentified previously. For example, Gal-6-O-sulfation of ligands dramatically increases the binding of Siglec-1 (32 vs 39 and 40, 34 vs 45 and 46, and 35 vs 48 and 49). GalNAc-6-O sulfation, rather than Gal-6-O sulfation, of Neu5Acα2,3Galβ1,3GalNAc resulted in an increased binding affinity to Siglec-4 (47 vs 48). Interestingly, despite the negatively charged nature of both sulfate and sialic acid, the replacement of GalNAc-6-O-sulfation with sialylation (49 to 50) resulted in a higher binding affinity to Siglec-1. In contrast to Siglec-1, the replacement of GalNAc-6-O-sulfation by sialylation caused a dramatic loss of Siglec-4 binding.
Likewise, sulfation also plays a critical role in fine-tuning the ligand binding affinity to Siglec-10. But unlike the above observations, recognition of the sulfated ligands by Siglec-10 is less affected by the types of a sugar residue at the reducing end and the linkages of either β1,3 or β1,4 of LacNAc. 6-O-Sulfation of the internal GlcNAc, Glc, or GalNAc residues of α2,6-linked sialosides 27, 28, 29, and 30 resulted in a significant increase in Siglec-10 binding ability (27, 28, 29, and 30 vs 23, 24, 25, and 26). However, for α2,3-linked sialosides (32, 33, 34, and 35), the effect of 6-O-sulfation on Siglec-10 binding more depends on the β1,3-linked backbone structure. For example, sulfation of ligand 32 with the Type II LacNAc backbone showed no significant gain in binding (38, 39, and 40); whereas sulfation of ligand 34 with the Type I LacNAc backbone significantly enhanced binding to Siglec-10 (44, 45, and 46).
Finally, it is worth pointing out that ligands presented in our array did not show strong binding to other Siglecs, including Siglec-5, -6, and -15 (Figure 8). The strongest signals are close to the background level. This is probably because the epitopes are too small to bind these Siglecs or the absence of specific glycan carriers for these Siglecs. Hence, installation of these epitopes on glycolipidsor glycoproteins may increase the binding signals. Although these bindings are weak, the binding specificity can be summarized. For example, disulfated SLex (53) is the dominant ligand for Siglec-15.
Conclusions
Operating orthogonally to the well-established immune checkpoints, PD-1 and CTLA-4,80 Siglecs have stimulated enthusiasm for developing strategies to suppress their inhibitory functions for cancer immunotherapy or to harness such function for the treatment of autoimmunity or inflammation. However, the ligand specificity has only been explored for a subfamily of Siglecs. In this study, we successfully developed a strategy using sialylation as a protecting approach for the rapid and efficient preparation of structure-defined glycan epitopes with site-specific sulfation. Using this strategy, 66 sulfated glycans with structures covering a large number of terminal sialylated glycan epitopes were prepared, allowing us to perform a comprehensive characterization of the ligand specificity for the entire Siglec family. Although our strategy is highly efficient for the preparation of sulfated glycan epitopes bearing disaccharide or trisaccharide backbones, it provides low yields when applied to the synthesis of glycans containing four or more monosaccharides, as the regio-specificity of the chemical sulfation reaction decreases.
Our screening revealed that not only sialylation and sulfation patterns but also the backbone structure of the ligands can influence recognition by Siglecs. We confirmed the glycan-binding patterns of Siglec-2, -3, -7, -8, and -9, which are consistent with those reported previously. In addition, our screening also revealed ligand specificity of less-studied Siglec-1, -4, -10, and -11 as well as subtle differences in ligand preference for several other Siglecs. For example, Siglec-4 prefers GalNAc-6-O sulfation, rather than Gal-6-O sulfation, of Neu5Acα2,3Galβ1,3GalNAc. It is worth noting that the α2,8 linkage of sialylation and sulfation of 6-O-Gal has a synergic effect on Siglec-7 binding. Siglec-8, but not Siglec-3, prefers disulfated glycans with the β1,3Gal backbone. Taken together, our study paves the way for the development of new Siglec-target strategies by providing a clear understanding of how sulfation and sialylation patterns govern the binding specificities of human Siglecs at the molecular level.
Acknowledgments
L.W. acknowledged the support from the Talent Plan of Shanghai Branch, Chinese Academy of Sciences (CASSHB-QNPD-2023-018), National Natural Science Foundation of China (92478106), Natural Science Foundation of Shanghai Municipality (22ZR1474000), and Shanghai Municipal Science and Technology Major Project. P.W. is partially supported by the NIH (R01AI154138). J.Z. is supported by the National Natural Science Foundation of China (22207113).
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/jacs.4c08817.
Additional experimental details, materials, and methods; and supporting figures, supporting table, 1H NMR spectra, and 13C NMR spectra for all compounds (PDF)
Author Contributions
∇ S. M., P. Z., and J. Y. contributed equally to this work.
The authors declare the following competing financial interest(s): The authors have submitted a patent application (China patent/PCT 2024113874483) regarding this work.
Supplementary Material
References
- Muthana S. M.; Campbell C. T.; Gildersleeve J. C. Modifications of Glycans: Biological Significance and Therapeutic Opportunities. ACS Chem. Biol. 2012, 7 (1), 31–43. 10.1021/cb2004466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luis A. S.; Baslé A.; Byrne D. P.; Wright G. S. A.; London J. A.; Jin C.; Karlsson N. G.; Hansson G. C.; Eyers P. A.; Czjzek M.; Barbeyron T.; Yates E. A.; Martens E. C.; Cartmell A. Sulfated glycan recognition by carbohydrate sulfatases of the human gut microbiota. Nat. Chem. Biol. 2022, 18 (8), 841–849. 10.1038/s41589-022-01039-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto K.; Ozaki T.; Ko Y.-C.; Tsai C.-F.; Gong Y.; Morozumi M.; Ishikawa Y.; Uchimura K.; Nadanaka S.; Kitagawa H.; Zulueta M. M. L.; Bandaru A.; Tamura J.-i.; Hung S.-C.; Kadomatsu K. Glycan sulfation patterns define autophagy flux at axon tip via PTPRσ-cortactin axis. Nat. Chem. Biol. 2019, 15 (7), 699–709. 10.1038/s41589-019-0274-x. [DOI] [PubMed] [Google Scholar]
- Kawashima H.; Petryniak B.; Hiraoka N.; Mitoma J.; Huckaby V.; Nakayama J.; Uchimura K.; Kadomatsu K.; Muramatsu T.; Lowe J. B.; Fukuda M. N-acetylglucosamine-6-O-sulfotransferases 1 and 2 cooperatively control lymphocyte homing through L-selectin ligand biosynthesis in high endothelial venules. Nat. Immunol. 2005, 6 (11), 1096–1104. 10.1038/ni1259. [DOI] [PubMed] [Google Scholar]
- Pröpster J. M.; Yang F.; Rabbani S.; Ernst B.; Allain F. H. T.; Schubert M. Structural basis for sulfation-dependent self-glycan recognition by the human immune-inhibitory receptor Siglec-8. Proc. Natl. Acad. Sci. U.S.A. 2016, 113 (29), E4170–E4179. 10.1073/pnas.1602214113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung J.; Enterina J. R.; Bui D. T.; Mozaneh F.; Lin P. H.; Nitin; Kuo C. W.; Rodrigues E.; Bhattacherjee A.; Raeisimakiani P.; Daskhan G. C.; St Laurent C. D.; Khoo K. H.; Mahal L. K.; Zandberg W. F.; Huang X. F.; Klassen J. S.; Macauley M. S.; et al. Carbohydrate Sulfation As a Mechanism for Fine-Tuning Siglec Ligands. ACS Chem. Biol. 2021, 16 (11), 2673–2689. 10.1021/acschembio.1c00501. [DOI] [PubMed] [Google Scholar]
- Dutta S.; Aoki K.; Doungkamchan K.; Tiemeyer M.; Bovin N.; Miller D. J. Sulfated Lewis A trisaccharide on oviduct membrane glycoproteins binds bovine sperm and lengthens sperm lifespan. J. Biol. Chem. 2019, 294 (36), 13445–13463. 10.1074/jbc.RA119.007695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández-Vega I.; García O.; Crespo A.; Castañón S.; Menéndez P.; Astudillo A.; Quirós L. M. Specific genes involved in synthesis and editing of heparan sulfate proteoglycans show altered expression patterns in breast cancer. BMC Cancer 2013, 13, 24. 10.1186/1471-2407-13-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plaas A. H. K.; West L. A.; Wong-Palms S.; Nelson F. R. T. Glycosaminoglycan Sulfation in Human Osteoarthritis. J. Biol. Chem. 1998, 273 (20), 12642–12649. 10.1074/jbc.273.20.12642. [DOI] [PubMed] [Google Scholar]
- Bayliss M. T.; Osborne D.; Woodhouse S.; Davidson C. Sulfation of chondroitin sulfate in human articular cartilage: the effect of age, topographical position, and zone of cartilage on tissue composition. J. Biol. Chem. 1999, 274 (22), 15892–15900. 10.1074/jbc.274.22.15892. [DOI] [PubMed] [Google Scholar]
- Sasisekharan R.; Shriver Z.; Venkataraman G.; Narayanasami U. Roles of heparan-sulphate glycosaminoglycans in cancer. Nat. Rev. Cancer 2002, 2 (7), 521–528. 10.1038/nrc842. [DOI] [PubMed] [Google Scholar]
- Soares da Costa D.; Reis R. L.; Pashkuleva I. Sulfation of Glycosaminoglycans and Its Implications in Human Health and Disorders. Annu. Rev. Biomed. Eng. 2017, 19 (1), 1–26. 10.1146/annurev-bioeng-071516-044610. [DOI] [PubMed] [Google Scholar]
- Crocker P. R.; Paulson J. C.; Varki A. Siglecs and their roles in the immune system. Nat. Rev. Immunol. 2007, 7 (4), 255–266. 10.1038/nri2056. [DOI] [PubMed] [Google Scholar]
- Macauley M. S.; Crocker P. R.; Paulson J. C. Siglec-mediated regulation of immune cell function in disease. Nat. Rev. Immunol. 2014, 14 (10), 653–666. 10.1038/nri3737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murugesan G.; Weigle B.; Crocker P. R. Siglec and anti-Siglec therapies. Curr. Opin. Chem. Biol. 2021, 62, 34–42. 10.1016/j.cbpa.2021.01.001. [DOI] [PubMed] [Google Scholar]
- Yu Y. Y.; Peng W. J. Recent progress in targeting the sialylated glycan-SIGLEC axis in cancer immunotherapy. Cancer Biol. Med. 2023, 20 (5), 369–384. 10.20892/j.issn.2095-3941.2023.0046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan S. T.; Paulson J. C. Siglecs as Immune Cell Checkpoints in Disease. Annu. Rev. Immunol. 2020, 38, 365–395. 10.1146/annurev-immunol-102419-035900. [DOI] [PubMed] [Google Scholar]
- Movsisyan L. D.; Macauley M. S. Structural advances of Siglecs: insight into synthetic glycan ligands for immunomodulation. Org. Biomol. Chem. 2020, 18 (30), 5784–5797. 10.1039/D0OB01116A. [DOI] [PubMed] [Google Scholar]
- Kornepati A. V. R.; Vadlamudi R. K.; Curiel T. J. Programmed death ligand 1 signals in cancer cells. Nat. Rev. Cancer 2022, 22 (3), 174–189. 10.1038/s41568-021-00431-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J.; Sun J.; Liu L. N.; Flies D. B.; Nie X.; Toki M.; Zhang J.; Song C.; Zarr M.; Zhou X.; Han X.; Archer K. A.; O’Neill T.; Herbst R. S.; Boto A. N.; Sanmamed M. F.; Langermann S.; Rimm D. L.; Chen L. Siglec-15 as an immune suppressor and potential target for normalization cancer immunotherapy. Nat. Med. 2019, 25 (4), 656–666. 10.1038/s41591-019-0374-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X. C.; Hanes M. S.; Cummings R. D.; Woods R. J. Computationally guided conversion of the specificity of E-selectin to mimic that of Siglec-8. Proc. Natl. Acad. Sci. U.S.A. 2022, 119 (41), e2117743119 10.1073/pnas.2117743119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Büll C.; Nason R.; Sun L.; Van Coillie J.; Madriz Sørensen D.; Moons S. J.; Yang Z.; Arbitman S.; Fernandes S. M.; Furukawa S.; McBride R.; Nycholat C. M.; Adema G. J.; Paulson J. C.; Schnaar R. L.; Boltje T. J.; Clausen H.; Narimatsu Y. Probing the binding specificities of human Siglecs by cell-based glycan arrays. Proc. Natl. Acad. Sci. U.S.A. 2021, 118 (17), e2026102118 10.1073/pnas.2026102118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campanero-Rhodes M. A.; Childs R. A.; Kiso M.; Komba S.; Le Narvor C.; Warren J.; Otto D.; Crocker P. R.; Feizi T. Carbohydrate microarrays reveal sulphation as a modulator of siglec binding. Biochem. Biophys. Res. Commun. 2006, 344 (4), 1141–1146. 10.1016/j.bbrc.2006.03.223. [DOI] [PubMed] [Google Scholar]
- Spence S.; Greene M. K.; Fay F.; Hams E.; Saunders S. P.; Hamid U.; Fitzgerald M.; Beck J.; Bains B. K.; Smyth P.; Themistou E.; Small D. M.; Schmid D.; O’Kane C. M.; Fitzgerald D. C.; Abdelghany S. M.; Johnston J. A.; Fallon P. G.; Burrows J. F.; McAuley D. F.; Kissenpfennig A.; Scott C. J. Targeting Siglecs with a sialic acid–decorated nanoparticle abrogates inflammation. Sci. Transl. Med. 2015, 7 (303), 303ra140. 10.1126/scitranslmed.aab3459. [DOI] [PubMed] [Google Scholar]
- Nycholat C. M.; Duan S. T.; Knuplez E.; Worth C.; Elich M.; Yao A. Z.; O’Sullivan J.; McBride R.; Wei Y. D.; Fernandes S. M.; Zhu Z.; Schnaar R. L.; Bochner B. S.; Paulson J. C. A Sulfonamide Sialoside Analogue for Targeting Siglec-8 and -F on Immune Cells. J. Am. Chem. Soc. 2019, 141 (36), 14032–14037. 10.1021/jacs.9b05769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W. C.; Completo G. C.; Sigal D. S.; Crocker P. R.; Saven A.; Paulson J. C. In vivo targeting of B-cell lymphoma with glycan ligands of CD22. Blood 2010, 115 (23), 4778–4786. 10.1182/blood-2009-12-257386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith B. A. H.; Bertozzi C. R. The clinical impact of glycobiology: targeting selectins, Siglecs and mammalian glycans. Nat. Rev. Drug Discovery 2021, 20 (3), 217–243. 10.1038/s41573-020-00093-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenza M. P.; Atxabal U.; Nycholat C.; Oyenarte I.; Franconetti A.; Quintana J. I.; Delgado S.; Núñez-Franco R.; Garnica Marroquín C. T.; Coelho H.; Unione L.; Jiménez-Oses G.; Marcelo F.; Schubert M.; Paulson J. C.; Jiménez-Barbero J.; Ereño-Orbea J. Structures of the Inhibitory Receptor Siglec-8 in Complex with a High-Affinity Sialoside Analogue and a Therapeutic Antibody. JACS Au 2023, 3 (1), 204–215. 10.1021/jacsau.2c00592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rillahan C. D.; Macauley M. S.; Schwartz E.; He Y.; McBride R.; Arlian B. M.; Rangarajan J.; Fokin V. V.; Paulson J. C. Disubstituted sialic acid ligands targeting siglecs CD33 and CD22 associated with myeloid leukaemias and B cell lymphomas. Chem. Sci. 2014, 5 (6), 2398–2406. 10.1039/c4sc00451e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenza M. P.; Egia-Mendikute L.; Antoñana-Vildosola A.; Soares C. O.; Coelho H.; Corzana F.; Bosch A.; Manisha P.; Quintana J. I.; Oyenarte I.; Unione L.; Moure M. J.; Azkargorta M.; Atxabal U.; Sobczak K.; Elortza F.; Sutherland J. D.; Barrio R.; Marcelo F.; Jiménez-Barbero J.; Palazon A.; Ereño-Orbea J. Structural insights into Siglec-15 reveal glycosylation dependency for its interaction with T cells through integrin CD11b. Nat. Commun. 2023, 14 (1), 3496. 10.1038/s41467-023-39119-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahm H. S.; Broecker F.; Kawasaki F.; Mietzsch M.; Heilbronn R.; Fukuda M.; Seeberger P. H. Automated Glycan Assembly of Oligo-N-Acetyllactosamine and Keratan Sulfate Probes to Study Virus-Glycan Interactions. Chem 2017, 2 (1), 114–124. 10.1016/j.chempr.2016.12.004. [DOI] [Google Scholar]
- Tyrikos-Ergas T.; Sletten E. T.; Huang J. Y.; Seeberger P. H.; Delbianco M. On resin synthesis of sulfated oligosaccharides. Chem. Sci. 2022, 13 (7), 2115–2120. 10.1039/D1SC06063E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L.; Sorum A. W.; Huang B.-S.; Kern M. K.; Su G.; Pawar N.; Huang X.; Liu J.; Pohl N. L. B.; Hsieh-Wilson L. C. Efficient platform for synthesizing comprehensive heparan sulfate oligosaccharide libraries for decoding glycosaminoglycan–protein interactions. Nat. Chem. 2023, 15 (8), 1108–1117. 10.1038/s41557-023-01248-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratt M. R.; Bertozzi C. R. Syntheses of 6-Sulfo Sialyl Lewis X Glycans Corresponding to the l-Selectin Ligand “Sulfoadhesin. Org. Lett. 2004, 6 (14), 2345–2348. 10.1021/ol0493195. [DOI] [PubMed] [Google Scholar]
- Al-Horani R. A.; Desai U. R. Chemical sulfation of small molecules—advances and challenges. Tetrahedron 2010, 66 (16), 2907–2918. 10.1016/j.tet.2010.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santra A.; Yu H.; Tasnima N.; Muthana M. M.; Li Y. H.; Zeng J.; Kenyon N. J.; Louie A. Y.; Chen X. Systematic chemoenzymatic synthesis of O-sulfated sialyl Lewis x antigens. Chem. Sci. 2016, 7 (4), 2827–2831. 10.1039/C5SC04104J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao T.; Yan J. Y.; Liu C. C.; Palma A. S.; Guo Z. M.; Xiao M.; Chen X.; Liang X. M.; Chai W. G.; Cao H. Z. Chemoenzymatic Synthesis of O-Mannose Glycans Containing Sulfated or Nonsulfated HNK-1 Epitope. J. Am. Chem. Soc. 2019, 141 (49), 19351–19359. 10.1021/jacs.9b08964. [DOI] [PubMed] [Google Scholar]
- Huang K.; Li C.; Zong G. H.; Prabhu S. K.; Chapla D. G.; Moremen K. W.; Wang L. X. Site-selective sulfation of N-glycans by human GlcNAc-6-O-sulfotransferase 1 (CHST2) and chemoenzymatic synthesis of sulfated antibody glycoforms. Bioorg. Chem. 2022, 128, 106070. 10.1016/j.bioorg.2022.106070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y.; Vos G. M.; Huang C.; Chapla D.; Kimpel A. L. M.; Moremen K. W.; de Vries R. P.; Boons G.-J. Exploiting Substrate Specificities of 6-O-Sulfotransferases to Enzymatically Synthesize Keratan Sulfate Oligosaccharides. JACS Au 2023, 3 (11), 3155–3164. 10.1021/jacsau.3c00488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y.; Bosman G. P.; Chapla D.; Huang C.; Moremen K. W.; de Vries R. P.; Boons G. J. A Biomimetic Synthetic Strategy Can Provide Keratan Sulfate I and II Oligosaccharides with Diverse Fucosylation and Sulfation Patterns. J. Am. Chem. Soc. 2024, 146 (13), 9230–9240. 10.1021/jacs.4c00363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moremen K. W.; Ramiah A.; Stuart M.; Steel J.; Meng L.; Forouhar F.; Moniz H. A.; Gahlay G.; Gao Z.; Chapla D.; Wang S.; Yang J. Y.; Prabhakar P. K.; Johnson R.; Rosa M. D.; Geisler C.; Nairn A. V.; Seetharaman J.; Wu S. C.; Tong L.; Gilbert H. J.; LaBaer J.; Jarvis D. L. Expression system for structural and functional studies of human glycosylation enzymes. Nat. Chem. Biol. 2018, 14 (2), 156–162. 10.1038/nchembio.2539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings R. D. The repertoire of glycan determinants in the human glycome. Mol. BioSyst. 2009, 5 (10), 1087–1104. 10.1039/b907931a. [DOI] [PubMed] [Google Scholar]
- Lau K.; Thon V.; Yu H.; Ding L.; Chen Y.; Muthana M. M.; Wong D.; Huang R.; Chen X. Highly efficient chemoenzymatic synthesis of β1–4-linked galactosides with promiscuous bacterial β1–4-galactosyltransferases. Chem. Commun. 2010, 46 (33), 6066–6068. 10.1039/c0cc01381a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sablina M. A.; Tuzikov A. B.; Ovchinnikova T. V.; Mikhura I. V.; Bovin N. V. Synthesis of mono- and di-O-sulfates of spacer-armed lactose. Russ. Chem. Bull. 2015, 64 (5), 1125–1133. 10.1007/s11172-015-0989-0. [DOI] [Google Scholar]
- Li Y. H.; Yu H.; Cao H. Z.; Muthana S.; Chen X. Pasteurella multocida CMP-sialic acid synthetase and mutants of Neisseria meningitidis CMP-sialic acid synthetase with improved substrate promiscuity. Appl. Microbiol. Biotechnol. 2012, 93 (6), 2411–2423. 10.1007/s00253-011-3579-6. [DOI] [PubMed] [Google Scholar]
- Yu H.; Huang S.; Chokhawala H.; Sun M.; Zheng H.; Chen X. Highly Efficient Chemoenzymatic Synthesis of Naturally Occurring and Non-Natural α-2,6-Linked Sialosides: A P. damsela α-2,6-Sialyltransferase with Extremely Flexible Donor–Substrate Specificity. Angew. Chem., Int. Ed. 2006, 45 (24), 3938–3944. 10.1002/anie.200600572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian Y. P.; Wang Y. Q.; Yin H. B.; Luo Y. W.; Wei F. Y.; Zhou H.; Wen L. Q. A Sensitive and Reversible Labeling Strategy Enables Global Mapping of the Core-Fucosylated Glycoproteome on Cell Surfaces. Angew. Chem., Int. Ed. 2022, 61 (49), e202206802 10.1002/anie.202206802. [DOI] [PubMed] [Google Scholar]
- Luo Y. W.; Wang Y. Q.; Tian Y. P.; Zhou H.; Wen L. Q. Two Birds One Stone″ Strategy for the Site-Specific Analysis of Core Fucosylation and O-GlcNAcylation. J. Am. Chem. Soc. 2023, 145 (29), 15879–15887. 10.1021/jacs.3c02976. [DOI] [PubMed] [Google Scholar]
- Xu G. G.; Kiefel M. J.; Wilson J. C.; Andrew P. W.; Oggioni M. R.; Taylor G. L. Three Streptococcus pneumoniae Sialidases: Three Different Products. J. Am. Chem. Soc. 2011, 133 (6), 1718–1721. 10.1021/ja110733q. [DOI] [PubMed] [Google Scholar]
- Mbua N. E.; Li X.; Flanagan-Steet H. R.; Meng L.; Aoki K.; Moremen K. W.; Wolfert M. A.; Steet R.; Boons G. J. Selective Exo-Enzymatic Labeling of N-Glycans on the Surface of Living Cells by Recombinant ST6Gal I. Angew. Chem., Int. Ed. 2013, 52 (49), 13012–13015. 10.1002/anie.201307095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen L.; Liu D.; Zheng Y.; Huang K.; Cao X.; Song J.; Wang P. G. A One-Step Chemoenzymatic Labeling Strategy for Probing Sialylated Thomsen–Friedenreich Antigen. ACS Cent. Sci. 2018, 4 (4), 451–457. 10.1021/acscentsci.7b00573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chokhawala H. A.; Huang S.; Lau K.; Yu H.; Cheng J.; Thon V.; Hurtado-Ziola N.; Guerrero J. A.; Varki A.; Chen X. Combinatorial Chemoenzymatic Synthesis and High-Throughput Screening of Sialosides. ACS Chem. Biol. 2008, 3 (9), 567–576. 10.1021/cb800127n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu Z.; Hsieh H. W.; Tsai C. M.; Hsu C. W.; Wang S. G.; Wu K. J.; Lin K. I.; Lin C. H. Synthesis and Characterization of Sulfated Gal-β-1,3/4-GlcNAc Disaccharides through Consecutive Protection/Glycosylation Steps. Chem.—Asian J. 2013, 8 (7), 1536–1550. 10.1002/asia.201201204. [DOI] [PubMed] [Google Scholar]
- Pazynina G.; Sablina M.; Ovchinnikova T.; Tyrtysh T.; Tsygankova S.; Tuzikov A.; Dobrochaeva K.; Shilova N.; Khasbiullina N.; Bovin N. Synthetic glyco-O-sulfatome for profiling of human natural antibodies. Carbohydr. Res. 2017, 445, 23–31. 10.1016/j.carres.2017.03.015. [DOI] [PubMed] [Google Scholar]
- Talafová K.; Hrabárová E.; Nahálka J. A semi-multifunctional sialyltransferase from Bibersteinia trehalosi and its comparison to the Pasteurella multocida ST1 mutants. J. Biotechnol. 2015, 216, 116–124. 10.1016/j.jbiotec.2015.09.031. [DOI] [PubMed] [Google Scholar]
- Wei F.; Zang L.; Zhang P.; Zhang J.; Wen L. Concise chemoenzymatic synthesis of N-glycans. Chem 2024, 10 (9), 2844–2860. 10.1016/j.chempr.2024.05.006. [DOI] [Google Scholar]
- Blanas A.; Sahasrabudhe N. M.; Rodríguez E.; van Kooyk Y.; van Vliet S. J. Fucosylated Antigens in Cancer: An Alliance toward Tumor Progression, Metastasis, and Resistance to Chemotherapy. Front. Oncol. 2018, 8, 39. 10.3389/fonc.2018.00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasko D. A.; Wang G.; Palcic M. M.; Taylor D. E. Cloning and Characterization of the α(1,3/4) Fucosyltransferase of Helicobacter pylori. J. Biol. Chem. 2000, 275 (7), 4988–4994. 10.1074/jbc.275.7.4988. [DOI] [PubMed] [Google Scholar]
- Mertsch A.; He N.; Yi D.; Kickstein M.; Fessner W. D. An α2,3-Sialyltransferase from Photobacterium phosphoreum with Broad Substrate Scope: Controlling Hydrolytic Activity by Directed Evolution. Chemistry 2020, 26 (50), 11614–11624. 10.1002/chem.202002277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwardt O.; Visekruna T.; Zenhäusern G.; Rabbani S.; Ernst B. Cloning, Expression, and Preparative Application of a Mutated, Bifunctional α(2→3/8)-Sialyltransferase from Campylobacter jejuni. J. Carbohydr. Chem. 2006, 25 (7), 543–556. 10.1080/07328300600966455. [DOI] [Google Scholar]
- Knibbs R. N.; Goldstein I. J.; Ratcliffe R. M.; Shibuya N. Characterization of the Carbohydrate Binding-Specificity of the Leukoagglutinating Lectin from Maackia-Amurensis - Comparison with Other Sialic Acid-Specific Lectins. J. Biol. Chem. 1991, 266 (1), 83–88. 10.1016/S0021-9258(18)52405-4. [DOI] [PubMed] [Google Scholar]
- Wang W. C.; Cummings R. D. The Immobilized Leukoagglutinin from the Seeds of Maackia-Amurensis Binds with High-Affinity to Complex-Type Asn-Linked Oligosaccharides Containing Terminal Sialic Acid-Linked Alpha-2,3 to Penultimate Galactose Residues. J. Biol. Chem. 1988, 263 (10), 4576–4585. 10.1016/S0021-9258(18)68821-0. [DOI] [PubMed] [Google Scholar]
- Smith D. F.; Song X. Z.; Cummings R. D. Use of Glycan Microarrays to Explore Specificity of Glycan-Binding Proteins. Methods Enzymol. 2010, 480, 417–444. 10.1016/S0076-6879(10)80033-3. [DOI] [PubMed] [Google Scholar]
- Song X. Z.; Yu H.; Chen X.; Lasanajak Y.; Tappert M. M.; Air G. M.; Tiwari V. K.; Cao H. Z.; Chokhawala H. A.; Zheng H. J.; Cummings R. D.; Smith D. F. A Sialylated Glycan Microarray Reveals Novel Interactions of Modified Sialic Acids with Proteins and Viruses. J. Biol. Chem. 2011, 286 (36), 31610–31622. 10.1074/jbc.M111.274217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blixt O.; Head S.; Mondala T.; Scanlan C.; Huflejt M. E.; Alvarez R.; Bryan M. C.; Fazio F.; Calarese D.; Stevens J.; Razi N.; Stevens D. J.; Skehel J. J.; van Die I.; Burton D. R.; Wilson I. A.; Cummings R.; Bovin N.; Wong C.-H.; Paulson J. C. Printed covalent glycan array for ligand profiling of diverse glycan binding proteins. Proc. Natl. Acad. Sci. U.S.A. 2004, 101 (49), 17033–17038. 10.1073/pnas.0407902101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotan R.; Skutelsky E.; Danon D.; Sharon N. The purification, composition, and specificity of the anti-T lectin from peanut (Arachis hypogaea). J. Biol. Chem. 1975, 250 (21), 8518–8523. 10.1016/S0021-9258(19)40790-4. [DOI] [PubMed] [Google Scholar]
- Bojar D.; Meche L.; Meng G. M.; Eng W.; Smith D. F.; Cummings R. D.; Mahal L. K. A Useful Guide to Lectin Binding: Machine-Learning Directed Annotation of 57 Unique Lectin Specificities. ACS Chem. Biol. 2022, 17 (11), 2993–3012. 10.1021/acschembio.1c00689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romano P. R.; Mackay A.; Vong M.; deSa J.; Lamontagne A.; Comunale M. A.; Hafner J.; Block T.; Lec R.; Mehta A. Development of recombinant Aleuria aurantia lectins with altered binding specificities to fucosylated glycans. Biochem. Biophys. Res. Commun. 2011, 414 (1), 84–89. 10.1016/j.bbrc.2011.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumura K.; Higashida K.; Hata Y.; Kominami J.; Nakamura-Tsuruta S.; Hirabayashi J. Comparative analysis of oligosaccharide specificities of fucose-specific lectins from Aspergillus oryzae and Aleuria aurantia using frontal affinity chromatography. Anal. Biochem. 2009, 386 (2), 217–221. 10.1016/j.ab.2008.11.044. [DOI] [PubMed] [Google Scholar]
- Mitra N.; Banda K.; Altheide T. K.; Schaffer L.; Johnson-Pais T. L.; Beuten J.; Leach R. J.; Angata T.; Varki N.; Varki A. SIGLEC12, a human-specific segregating (pseudo)gene, encodes a signaling molecule expressed in prostate carcinomas. J. Biol. Chem. 2011, 286 (26), 23003–23011. 10.1074/jbc.M111.244152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angata T.; Hayakawa T.; Yamanaka M.; Varki A.; Nakamura M. Discovery of Siglec-14, a novel sialic acid receptor undergoing concerted evolution with Siglec-5 in primates. FASEB J. 2006, 20 (12), 1964–1973. 10.1096/fj.06-5800com. [DOI] [PubMed] [Google Scholar]
- Ali S. R.; Fong J. J.; Carlin A. F.; Busch T. D.; Linden R.; Angata T.; Areschoug T.; Parast M.; Varki N.; Murray J.; Nizet V.; Varki A. Siglec-5 and Siglec-14 are polymorphic paired receptors that modulate neutrophil and amnion signaling responses to group B Streptococcus. J. Exp. Med. 2014, 211 (6), 1231–1242. 10.1084/jem.20131853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayakawa T.; Khedri Z.; Schwarz F.; Landig C.; Liang S. Y.; Yu H.; Chen X.; Fujito N. T.; Satta Y.; Varki A.; Angata T. Coevolution of Siglec-11 and Siglec-16 via gene conversion in primates. BMC Evol. Biol. 2017, 17, 228. 10.1186/s12862-017-1075-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Berg T. K.; Breve J. J.; Damoiseaux J. G.; Döpp E. A.; Kelm S.; Crocker P. R.; Dijkstra C. D.; Kraal G. Sialoadhesin on macrophages: its identification as a lymphocyte adhesion molecule. J. Exp. Med. 1992, 176 (3), 647–655. 10.1084/jem.176.3.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neill A. S.; van den Berg T. K.; Mullen G. E. Sialoadhesin - a macrophage-restricted marker of immunoregulation and inflammation. Immunology 2013, 138 (3), 198–207. 10.1111/imm.12042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng Y.; Rademacher C.; Nycholat C. M.; Futakawa S.; Lemme K.; Ernst B.; Paulson J. C. High affinity sialoside ligands of myelin associated glycoprotein. Bioorg. Med. Chem. Lett. 2011, 21 (17), 5045–5049. 10.1016/j.bmcl.2011.04.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forgione R. E.; Di Carluccio C.; Guzmán-Caldentey J.; Gaglione R.; Battista F.; Chiodo F.; Manabe Y.; Arciello A.; Del Vecchio P.; Fukase K.; Molinaro A.; Martín-Santamaría S.; Crocker P. R.; Marchetti R.; Silipo A. Unveiling Molecular Recognition of Sialoglycans by Human Siglec-10. iScience 2020, 23 (6), 101231. 10.1016/j.isci.2020.101231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blixt O.; Collins B. E.; van den Nieuwenhof I. M.; Crocker P. R.; Paulson J. C. Sialoside Specificity of the Siglec Family Assessed Using Novel Multivalent Probes. J. Biol. Chem. 2003, 278 (33), 31007–31019. 10.1074/jbc.M304331200. [DOI] [PubMed] [Google Scholar]
- Rapoport E.; Mikhalyov I.; Zhang J. Q.; Crocker P.; Bovin N. Ganglioside binding pattern of CD33-related Siglecs. Bioorg. Med. Chem. Lett. 2003, 13 (4), 675–678. 10.1016/S0960-894X(02)00998-8. [DOI] [PubMed] [Google Scholar]
- Buchbinder E. I.; Desai A. CTLA-4 and PD-1 Pathways Similarities, Differences, and Implications of Their Inhibition. Am. J. Clin. Oncol. 2016, 39 (1), 98–106. 10.1097/COC.0000000000000239. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.