Abstract
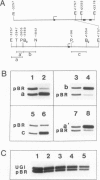
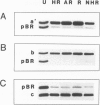
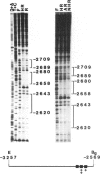
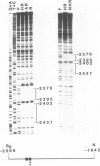
The effects of ligand binding and receptor phosphorylation on the interaction of progesterone receptor with specific DNA sequences in the uteroglobin gene were studied by nitro-cellulose filter binding and DNase I footprinting. High affinity sites were mapped upstream from the transcription start and in the first intron. They contained a common TGTTCACT sequence. These sites were occupied with similar affinity by the receptor, either in its free state, or complexed with the hormone or an antagonist (RU486); and also by receptor which had been phosphorylated in vivo in a hormone-dependent manner. In all cases identical footprints were observed. These experiments led to the following conclusions. The hormone-dependency of receptor binding to DNA or chromatin is observed in intact cells and in crude cellular extracts but not with purified receptor. Thus in situ, the unliganded receptor probably interacts with some nuclear component(s) which stabilizes it in a 'non-activated' form (non-chromatin and non-DNA binding form). When isolated, the receptor may undergo activation, even in the absence of the hormone. Binding by receptor of an antihormone (and possibly receptor phosphorylation) exerts an effect on gene transcription through a mechanism which is different from (and probably follows) receptor interaction with the gene.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atger M., Atger P., Tiollais P., Milgrom E. Cloning of rabbit genomic fragments containing the uteroglobin gene. J Biol Chem. 1981 Jun 25;256(12):5970–5972. [PubMed] [Google Scholar]
- Bailly A., Atger M., Atger P., Cerbon M. A., Alizon M., Vu Haï M. T., Logeat F., Milgrom E. The rabbit uteroglobin gene. Structure and interaction with the progesterone receptor. J Biol Chem. 1983 Sep 10;258(17):10384–10389. [PubMed] [Google Scholar]
- Bourgeois S., Pfahl M., Baulieu E. E. DNA binding properties of glucocorticosteroid receptors bound to the steroid antagonist RU-486. EMBO J. 1984 Apr;3(4):751–755. doi: 10.1002/j.1460-2075.1984.tb01879.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catelli M. G., Binart N., Jung-Testas I., Renoir J. M., Baulieu E. E., Feramisco J. R., Welch W. J. The common 90-kd protein component of non-transformed '8S' steroid receptors is a heat-shock protein. EMBO J. 1985 Dec 1;4(12):3131–3135. doi: 10.1002/j.1460-2075.1985.tb04055.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cato A. C., Geisse S., Wenz M., Westphal H. M., Beato M. The nucleotide sequences recognized by the glucocorticoid receptor in the rabbit uteroglobin gene region are located far upstream from the initiation of transcription. EMBO J. 1984 Dec 1;3(12):2771–2778. doi: 10.1002/j.1460-2075.1984.tb02208.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler V. L., Maler B. A., Yamamoto K. R. DNA sequences bound specifically by glucocorticoid receptor in vitro render a heterologous promoter hormone responsive in vivo. Cell. 1983 Jun;33(2):489–499. doi: 10.1016/0092-8674(83)90430-0. [DOI] [PubMed] [Google Scholar]
- Compton J. G., Schrader W. T., O'Malley B. W. DNA sequence preference of the progesterone receptor. Proc Natl Acad Sci U S A. 1983 Jan;80(1):16–20. doi: 10.1073/pnas.80.1.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean D. C., Knoll B. J., Riser M. E., O'Malley B. W. A 5'-flanking sequence essential for progesterone regulation of an ovalbumin fusion gene. Nature. 1983 Oct 6;305(5934):551–554. doi: 10.1038/305551a0. [DOI] [PubMed] [Google Scholar]
- Evans E., Baskevitch P. P., Rochefort H. Estrogen-receptor--DNA interaction. Difference between activation by estrogen and antiestrogen. Eur J Biochem. 1982 Nov;128(1):185–191. [PubMed] [Google Scholar]
- Galas D. J., Schmitz A. DNAse footprinting: a simple method for the detection of protein-DNA binding specificity. Nucleic Acids Res. 1978 Sep;5(9):3157–3170. doi: 10.1093/nar/5.9.3157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisse S., Scheidereit C., Westphal H. M., Hynes N. E., Groner B., Beato M. Glucocorticoid receptors recognize DNA sequences in and around murine mammary tumour virus DNA. EMBO J. 1982;1(12):1613–1619. doi: 10.1002/j.1460-2075.1982.tb01363.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govindan M. V., Spiess E., Majors J. Purified glucocorticoid receptor-hormone complex from rat liver cytosol binds specifically to cloned mouse mammary tumor virus long terminal repeats in vitro. Proc Natl Acad Sci U S A. 1982 Sep;79(17):5157–5161. doi: 10.1073/pnas.79.17.5157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes N., van Ooyen A. J., Kennedy N., Herrlich P., Ponta H., Groner B. Subfragments of the large terminal repeat cause glucocorticoid-responsive expression of mouse mammary tumor virus and of an adjacent gene. Proc Natl Acad Sci U S A. 1983 Jun;80(12):3637–3641. doi: 10.1073/pnas.80.12.3637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karin M., Haslinger A., Holtgreve H., Richards R. I., Krauter P., Westphal H. M., Beato M. Characterization of DNA sequences through which cadmium and glucocorticoid hormones induce human metallothionein-IIA gene. Nature. 1984 Apr 5;308(5959):513–519. doi: 10.1038/308513a0. [DOI] [PubMed] [Google Scholar]
- Logeat F., Le Cunff M., Pamphile R., Milgrom E. The nuclear-bound form of the progesterone receptor is generated through a hormone-dependent phosphorylation. Biochem Biophys Res Commun. 1985 Aug 30;131(1):421–427. doi: 10.1016/0006-291x(85)91819-4. [DOI] [PubMed] [Google Scholar]
- Logeat F., Pamphile R., Loosfelt H., Jolivet A., Fournier A., Milgrom E. One-step immunoaffinity purification of active progesterone receptor. Further evidence in favor of the existence of a single steroid binding subunit. Biochemistry. 1985 Feb 12;24(4):1029–1035. doi: 10.1021/bi00325a034. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Moore D. D., Marks A. R., Buckley D. I., Kapler G., Payvar F., Goodman H. M. The first intron of the human growth hormone gene contains a binding site for glucocorticoid receptor. Proc Natl Acad Sci U S A. 1985 Feb;82(3):699–702. doi: 10.1073/pnas.82.3.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulvihill E. R., LePennec J. P., Chambon P. Chicken oviduct progesterone receptor: location of specific regions of high-affinity binding in cloned DNA fragments of hormone-responsive genes. Cell. 1982 Mar;28(3):621–632. doi: 10.1016/0092-8674(82)90217-3. [DOI] [PubMed] [Google Scholar]
- Parker M. Enhancer elements activated by steroid hormones? Nature. 1983 Aug 25;304(5928):687–688. doi: 10.1038/304687a0. [DOI] [PubMed] [Google Scholar]
- Payvar F., DeFranco D., Firestone G. L., Edgar B., Wrange O., Okret S., Gustafsson J. A., Yamamoto K. R. Sequence-specific binding of glucocorticoid receptor to MTV DNA at sites within and upstream of the transcribed region. Cell. 1983 Dec;35(2 Pt 1):381–392. doi: 10.1016/0092-8674(83)90171-x. [DOI] [PubMed] [Google Scholar]
- Payvar F., Wrange O., Carlstedt-Duke J., Okret S., Gustafsson J. A., Yamamoto K. R. Purified glucocorticoid receptors bind selectively in vitro to a cloned DNA fragment whose transcription is regulated by glucocorticoids in vivo. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6628–6632. doi: 10.1073/pnas.78.11.6628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrot-Applanat M., Groyer-Picard M. T., Logeat F., Milgrom E. Ultrastructural localization of the progesterone receptor by an immunogold method: effect of hormone administration. J Cell Biol. 1986 Apr;102(4):1191–1199. doi: 10.1083/jcb.102.4.1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrot-Applanat M., Logeat F., Groyer-Picard M. T., Milgrom E. Immunocytochemical study of mammalian progesterone receptor using monoclonal antibodies. Endocrinology. 1985 Apr;116(4):1473–1484. doi: 10.1210/endo-116-4-1473. [DOI] [PubMed] [Google Scholar]
- Pfahl M. Specific binding of the glucocorticoid-receptor complex to the mouse mammary tumor proviral promoter region. Cell. 1982 Dec;31(2 Pt 1):475–482. doi: 10.1016/0092-8674(82)90140-4. [DOI] [PubMed] [Google Scholar]
- Pike J. W., Sleator N. M. Hormone-dependent phosphorylation of the 1,25-dihydroxyvitamin D3 receptor in mouse fibroblasts. Biochem Biophys Res Commun. 1985 Aug 30;131(1):378–385. doi: 10.1016/0006-291x(85)91813-3. [DOI] [PubMed] [Google Scholar]
- Rauch M., Loosfelt H., Philibert D., Milgrom E. Mechanism of action of an antiprogesterone, RU486, in the rabbit endometrium. Effects of RU486 on the progesterone receptor and on the expression of the uteroglobin gene. Eur J Biochem. 1985 Apr 15;148(2):213–218. doi: 10.1111/j.1432-1033.1985.tb08827.x. [DOI] [PubMed] [Google Scholar]
- Renkawitz R., Schütz G., von der Ahe D., Beato M. Sequences in the promoter region of the chicken lysozyme gene required for steroid regulation and receptor binding. Cell. 1984 Jun;37(2):503–510. doi: 10.1016/0092-8674(84)90380-5. [DOI] [PubMed] [Google Scholar]
- Robins D. M., Paek I., Seeburg P. H., Axel R. Regulated expression of human growth hormone genes in mouse cells. Cell. 1982 Jun;29(2):623–631. doi: 10.1016/0092-8674(82)90178-7. [DOI] [PubMed] [Google Scholar]
- Sanchez E. R., Toft D. O., Schlesinger M. J., Pratt W. B. Evidence that the 90-kDa phosphoprotein associated with the untransformed L-cell glucocorticoid receptor is a murine heat shock protein. J Biol Chem. 1985 Oct 15;260(23):12398–12401. [PubMed] [Google Scholar]
- Scheidereit C., Beato M. Contacts between hormone receptor and DNA double helix within a glucocorticoid regulatory element of mouse mammary tumor virus. Proc Natl Acad Sci U S A. 1984 May;81(10):3029–3033. doi: 10.1073/pnas.81.10.3029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheidereit C., Geisse S., Westphal H. M., Beato M. The glucocorticoid receptor binds to defined nucleotide sequences near the promoter of mouse mammary tumour virus. Nature. 1983 Aug 25;304(5928):749–752. doi: 10.1038/304749a0. [DOI] [PubMed] [Google Scholar]
- Slater E. P., Rabenau O., Karin M., Baxter J. D., Beato M. Glucocorticoid receptor binding and activation of a heterologous promoter by dexamethasone by the first intron of the human growth hormone gene. Mol Cell Biol. 1985 Nov;5(11):2984–2992. doi: 10.1128/mcb.5.11.2984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suske G., Wenz M., Cato A. C., Beato M. The uteroglobin gene region: hormonal regulation, repetitive elements and complete nucleotide sequence of the gene. Nucleic Acids Res. 1983 Apr 25;11(8):2257–2271. doi: 10.1093/nar/11.8.2257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland R. L., Murphy L. C. Mechanisms of oestrogen antagonism by nonsteroidal antioestrogens. Mol Cell Endocrinol. 1982 Jan;25(1):5–23. doi: 10.1016/0303-7207(82)90165-4. [DOI] [PubMed] [Google Scholar]
- Takeda Y., Ohlendorf D. H., Anderson W. F., Matthews B. W. DNA-binding proteins. Science. 1983 Sep 9;221(4615):1020–1026. doi: 10.1126/science.6308768. [DOI] [PubMed] [Google Scholar]
- von der Ahe D., Janich S., Scheidereit C., Renkawitz R., Schütz G., Beato M. Glucocorticoid and progesterone receptors bind to the same sites in two hormonally regulated promoters. Nature. 1985 Feb 21;313(6004):706–709. doi: 10.1038/313706a0. [DOI] [PubMed] [Google Scholar]