Abstract
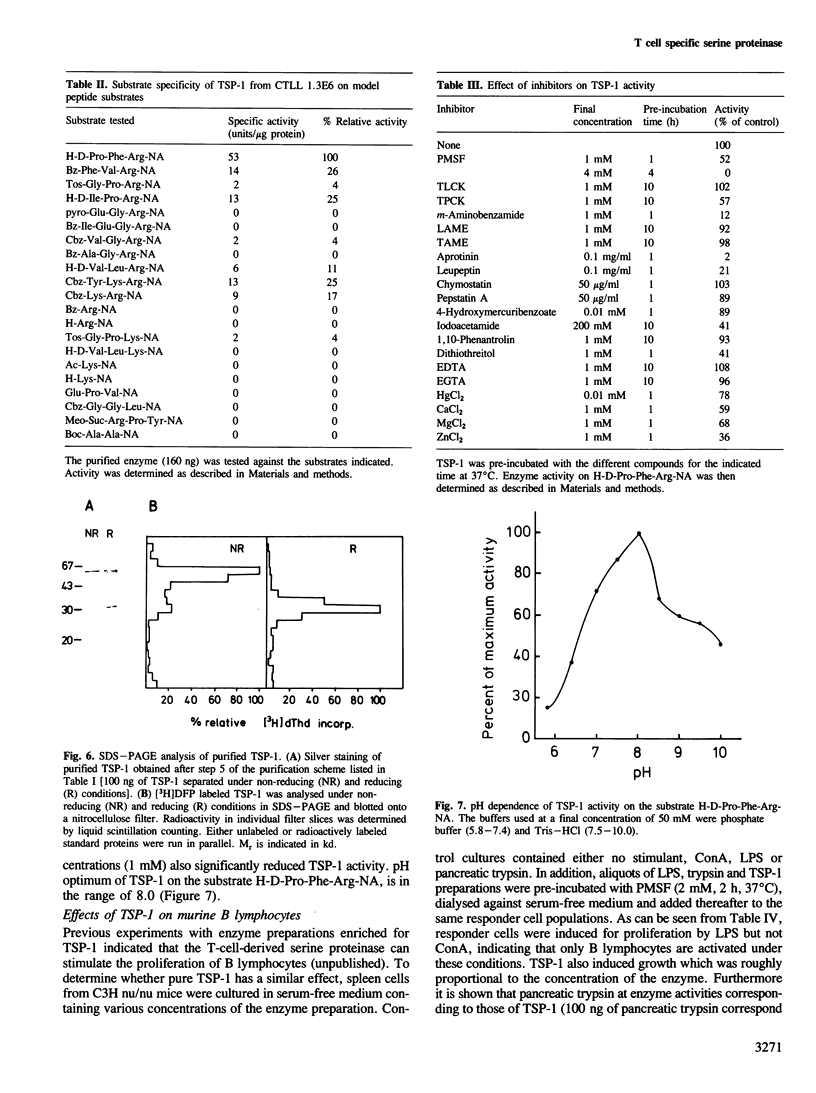
We describe the purification of a T cell specific serine proteinase derived from a cloned murine cytolytic T lymphocyte line. Analysis of the enzyme by sodium dodecyl sulfate polyacrylamide gel electrophoresis revealed a mol. wt of approximately 60 kd under non-reducing conditions and of approximately 30 kd under reducing conditions. The proteinase cleaves the model peptide substrate H-D-Pro-Phe-Arg-NA, at the 4 nitroanilide (NA) group with high efficiency. Much lower or no activity of the enzyme is found against synthetic peptide substrates carrying other amino acid (AA) sequences at position P2, P3 adjacent to L-arginine or against substrates in which AA other than L-arginine are bound to the NA group. Optimal pH for the cleavage of H-D-Pro-Phe-Arg-NA is in the range of 8.0-8.5. The enzyme is strongly inhibited by inhibitors of serine proteinases, diisopropylfluorophosphate, phenylmethane-sulfonyl fluoride, m-aminobenzamidine, aprotinin, and leupeptin but not by inhibitors of either thiol-, metallo- or carboxyl-proteinases. We propose the designation TSP-1 (T-cell derived serine proteinase 1) for this enzyme. TSP-1 has the capacity to stimulate B lymphocytes for proliferation in the absence of antigen.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Achstetter T., Wolf D. H. Proteinases, proteolysis and biological control in the yeast Saccharomyces cerevisiae. Yeast. 1985 Dec;1(2):139–157. doi: 10.1002/yea.320010203. [DOI] [PubMed] [Google Scholar]
- Ansorge W. Fast and sensitive detection of protein and DNA bands by treatment with potassium permanganate. J Biochem Biophys Methods. 1985 May;11(1):13–20. doi: 10.1016/0165-022x(85)90037-5. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brunet J. F., Dosseto M., Denizot F., Mattei M. G., Clark W. R., Haqqi T. M., Ferrier P., Nabholz M., Schmitt-Verhulst A. M., Luciani M. F. The inducible cytotoxic T-lymphocyte-associated gene transcript CTLA-1 sequence and gene localization to mouse chromosome 14. Nature. 1986 Jul 17;322(6076):268–271. doi: 10.1038/322268a0. [DOI] [PubMed] [Google Scholar]
- Chang T. W., Eisen H. N. Effects of N alpha-tosyl-L-lysyl-chloromethylketone on the activity of cytotoxic T lymphocytes. J Immunol. 1980 Mar;124(3):1028–1033. [PubMed] [Google Scholar]
- Gershenfeld H. K., Weissman I. L. Cloning of a cDNA for a T cell-specific serine protease from a cytotoxic T lymphocyte. Science. 1986 May 16;232(4752):854–858. doi: 10.1126/science.2422755. [DOI] [PubMed] [Google Scholar]
- Glenn K. C., Cunningham D. D. Thrombin-stimulated cell division involves proteolysis of its cell surface receptor. Nature. 1979 Apr 19;278(5706):711–714. doi: 10.1038/278711a0. [DOI] [PubMed] [Google Scholar]
- Goutner A., Simmler M. C., Tapon J., Rosenfeld C. Modulation by alpha-2 macroglobulin of human lymphocyte proliferation in response to mitogens and antigen. Differentiation. 1976 Jun 4;5(2-3):171–173. doi: 10.1111/j.1432-0436.1976.tb00910.x. [DOI] [PubMed] [Google Scholar]
- Henkart P. A., Millard P. J., Reynolds C. W., Henkart M. P. Cytolytic activity of purified cytoplasmic granules from cytotoxic rat large granular lymphocyte tumors. J Exp Med. 1984 Jul 1;160(1):75–93. doi: 10.1084/jem.160.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudig D., Redelman D., Minning L. L. The requirement for proteinase activity for human lymphocyte-mediated natural cytotoxicity (NK): evidence that the proteinase is serine dependent and has aromatic amino acid specificity of cleavage. J Immunol. 1984 Nov;133(5):2647–2654. [PubMed] [Google Scholar]
- Julius M. H., Simpson E., Herzenberg L. A. A rapid method for the isolation of functional thymus-derived murine lymphocytes. Eur J Immunol. 1973 Oct;3(10):645–649. doi: 10.1002/eji.1830031011. [DOI] [PubMed] [Google Scholar]
- Kaplan J. G., Bona C. Proteases as mitogens. The Effect of trypsin and pronase on mouse and human lymphocytes. Exp Cell Res. 1974 Oct;88(2):388–394. doi: 10.1016/0014-4827(74)90257-2. [DOI] [PubMed] [Google Scholar]
- Kramer M. D., Binninger L., Schirrmacher V., Moll H., Prester M., Nerz G., Simon M. M. Characterization and isolation of a trypsin-like serine protease from a long-term culture cytolytic T cell line and its expression by functionally distinct T cells. J Immunol. 1986 Jun 15;136(12):4644–4651. [PubMed] [Google Scholar]
- Kramer M. D., Robinson P., Vlodavsky I., Barz D., Friberger P., Fuks Z., Schirrmacher V. Characterization of an extracellular matrix-degrading protease derived from a highly metastatic tumor cell line. Eur J Cancer Clin Oncol. 1985 Mar;21(3):307–316. doi: 10.1016/0277-5379(85)90130-0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lavie G., Leib Z., Servadio C. The mechanism of human NK cell-mediated cytotoxicity. Mode of action of surface-associated proteases in the early stages of the lytic reaction. J Immunol. 1985 Aug;135(2):1470–1476. [PubMed] [Google Scholar]
- Lobe C. G., Finlay B. B., Paranchych W., Paetkau V. H., Bleackley R. C. Novel serine proteases encoded by two cytotoxic T lymphocyte-specific genes. Science. 1986 May 16;232(4752):858–861. doi: 10.1126/science.3518058. [DOI] [PubMed] [Google Scholar]
- Masson D., Nabholz M., Estrade C., Tschopp J. Granules of cytolytic T-lymphocytes contain two serine esterases. EMBO J. 1986 Jul;5(7):1595–1600. doi: 10.1002/j.1460-2075.1986.tb04401.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullins D. E., Rohrlich S. T. The role of proteinases in cellular invasiveness. Biochim Biophys Acta. 1983 Dec 29;695(3-4):177–214. doi: 10.1016/0304-419x(83)90011-2. [DOI] [PubMed] [Google Scholar]
- Pasternack M. S., Eisen H. N. A novel serine esterase expressed by cytotoxic T lymphocytes. 1985 Apr 25-May 1Nature. 314(6013):743–745. doi: 10.1038/314743a0. [DOI] [PubMed] [Google Scholar]
- Pasternack M. S., Verret C. R., Liu M. A., Eisen H. N. Serine esterase in cytolytic T lymphocytes. Nature. 1986 Aug 21;322(6081):740–743. doi: 10.1038/322740a0. [DOI] [PubMed] [Google Scholar]
- Podack E. R., Konigsberg P. J. Cytolytic T cell granules. Isolation, structural, biochemical, and functional characterization. J Exp Med. 1984 Sep 1;160(3):695–710. doi: 10.1084/jem.160.3.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redelman D., Hudig D. The mechanism of cell-mediated cytotoxicity. III. Protease-specific inhibitors preferentially block later events in cytotoxic T lymphocyte-mediated lysis than do inhibitors of methylation or thiol-reactive agents. Cell Immunol. 1983 Oct 1;81(1):9–21. doi: 10.1016/0008-8749(83)90206-x. [DOI] [PubMed] [Google Scholar]
- Simon M. M., Weltzien H. U., Bühring H. J., Eichmann K. Aged murine killer T-cell clones acquire specific cytotoxicity for P815 mastocytoma cells. Nature. 1984 Mar 22;308(5957):367–370. doi: 10.1038/308367a0. [DOI] [PubMed] [Google Scholar]
- Vischer T. L. Stimulation of mouse B lymphocytes by trypsin. J Immunol. 1974 Jul;113(1):58–62. [PubMed] [Google Scholar]


