Abstract
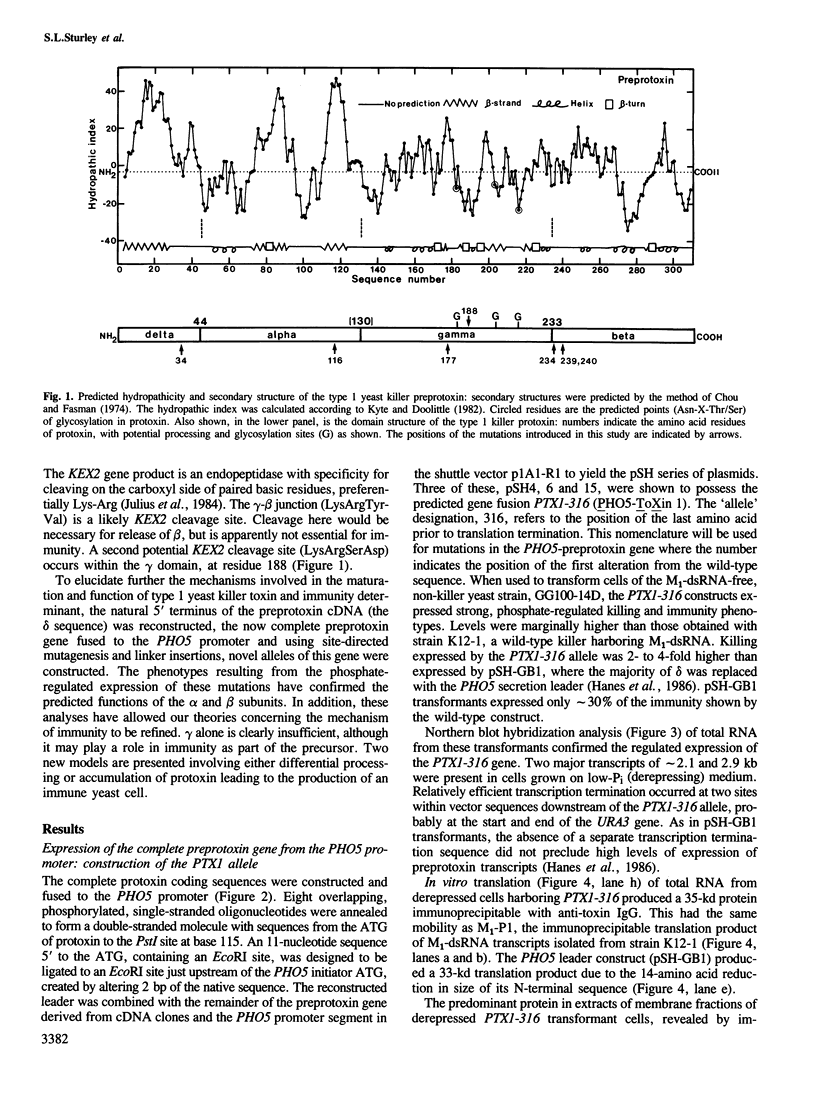
Strains of Saccharomyces cerevisiae harboring M1-dsRNA, the determinant of type 1 killer and immunity phenotypes, secrete a dimeric 19-kd toxin that kills sensitive yeast cells by the production of cation-permeable pores in the cytoplasmic membrane. The preprotoxin, an intracellular precursor to toxin, has the domain sequence delta-alpha-gamma-beta where alpha and beta are the 9.5-and 9.0-kd subunits of secreted toxin. Plasmids containing a partial cDNA copy of M1, in which alpha, gamma, and beta are fused to the PH05 promoter and signal peptide, have previously been shown to express phosphate-repressible toxin production and immunity. Here the construction of a complete DNA copy of the preprotoxin gene and its mutagenesis are described. Analysis of the expression of these mutants from the PH05 promoter elucidates the functions of the preprotoxin domains. delta acts as a leader peptide and efficiently mediates the secretion, glycosylation and maturation of killer toxin. Mutations within the beta subunit indicate it to be essential for binding of toxin to and killing of whole cells but unnecessary for the killing of spheroplasts. Mutations within the putative active site of alpha prevent killing of both cells and spheroplasts. The probable role of beta is therefore recognition and binding to the cell wall receptor whereas alpha is the active ionophore. Mutations within alpha causing loss of toxicity also cause loss of immunity, while the mutants described within gamma and beta retain partial or complete immunity. Expression of gamma without alpha or beta confers no phenotype. The immunity determinant may minimally consist of the alpha domain and the N-terminal portion of gamma.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF
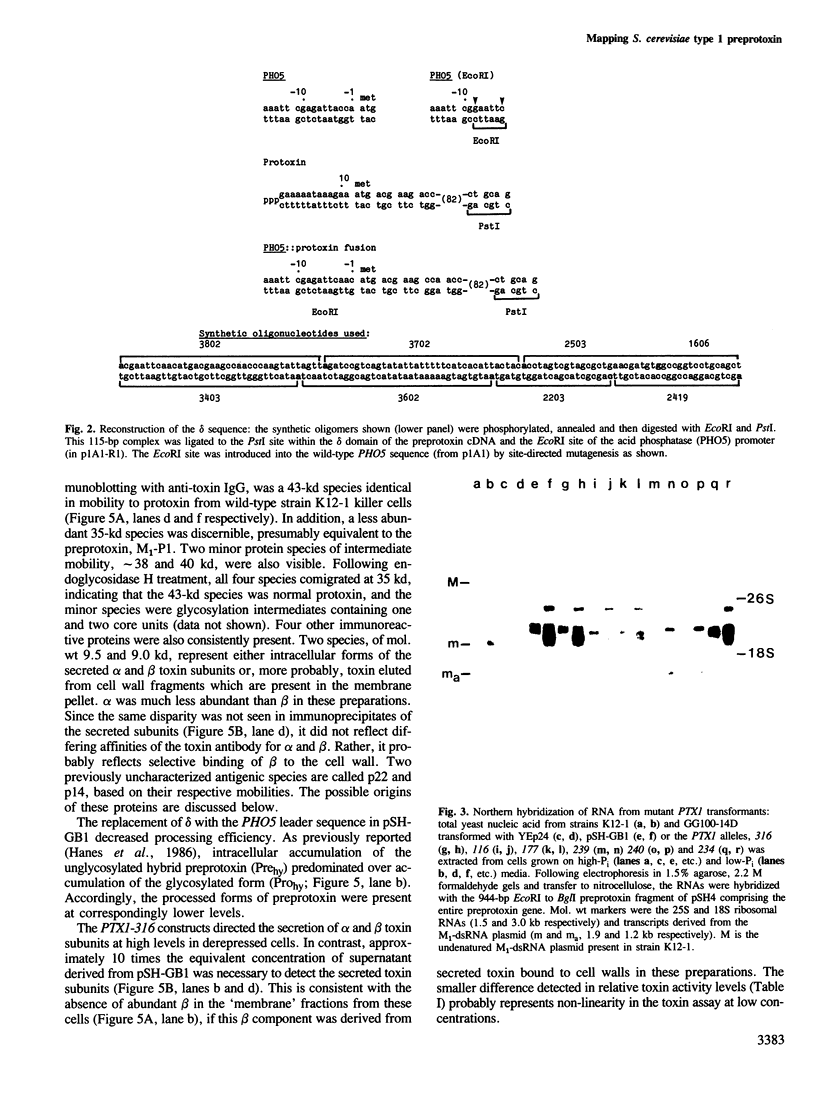
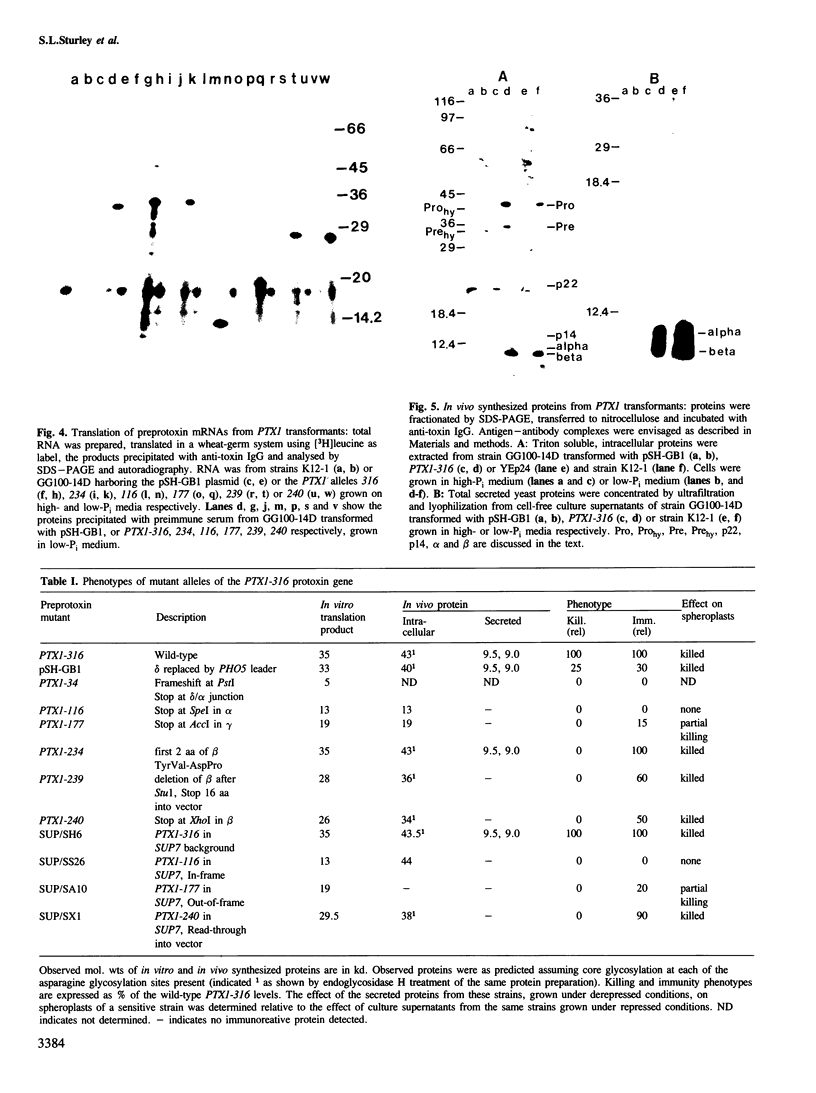
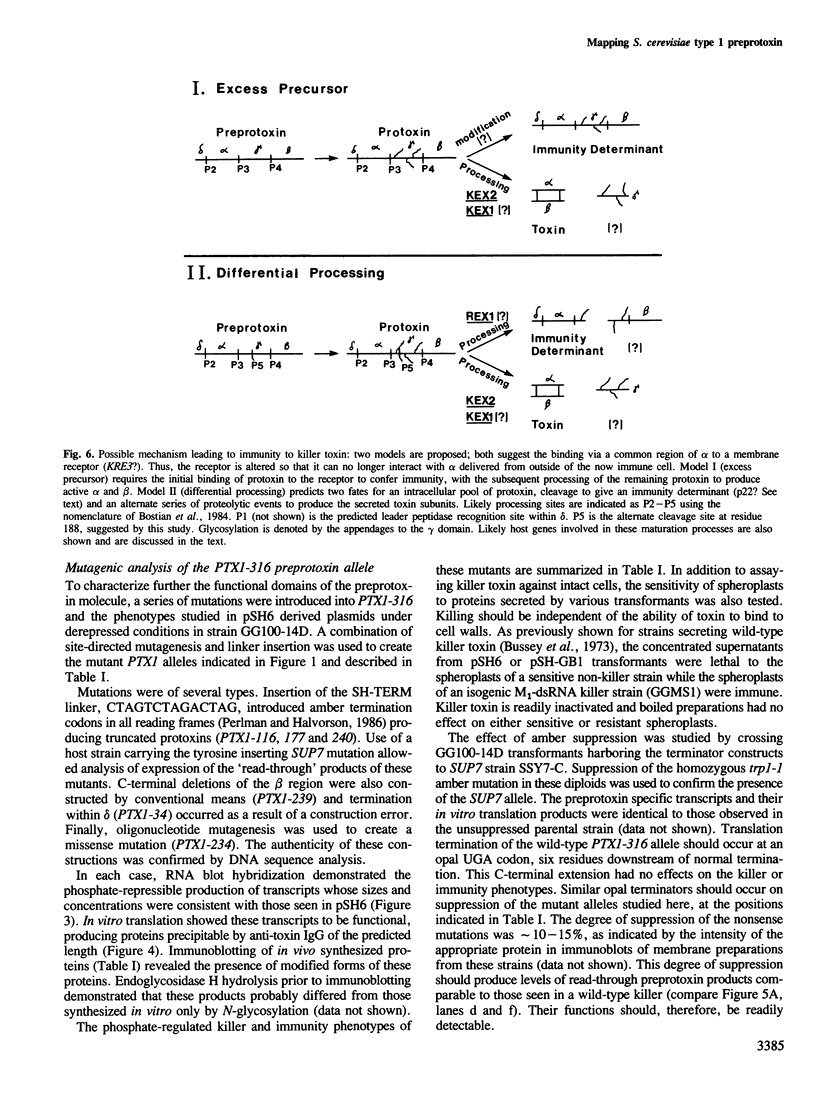




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Al-Aidroos K., Bussey H. Chromosomal mutants of Saccharomyces cerevisiae affecting the cell wall binding site for killer factor. Can J Microbiol. 1978 Mar;24(3):228–237. doi: 10.1139/m78-041. [DOI] [PubMed] [Google Scholar]
- Blake M. S., Johnston K. H., Russell-Jones G. J., Gotschlich E. C. A rapid, sensitive method for detection of alkaline phosphatase-conjugated anti-antibody on Western blots. Anal Biochem. 1984 Jan;136(1):175–179. doi: 10.1016/0003-2697(84)90320-8. [DOI] [PubMed] [Google Scholar]
- Bostian K. A., Burn V. E., Jayachandran S., Tipper D. J. Yeast killer dsRNA plasmids are transcribed in vivo to produce full and partial-length plus-stranded RNAs. Nucleic Acids Res. 1983 Feb 25;11(4):1077–1097. doi: 10.1093/nar/11.4.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bostian K. A., Elliott Q., Bussey H., Burn V., Smith A., Tipper D. J. Sequence of the preprotoxin dsRNA gene of type I killer yeast: multiple processing events produce a two-component toxin. Cell. 1984 Mar;36(3):741–751. doi: 10.1016/0092-8674(84)90354-4. [DOI] [PubMed] [Google Scholar]
- Bostian K. A., Hopper J. E., Rogers D. T., Tipper D. J. Translational analysis of the killer-associated virus-like particle dsRNA genome of S. cerevisiae: M dsRNA encodes toxin. Cell. 1980 Feb;19(2):403–414. doi: 10.1016/0092-8674(80)90514-0. [DOI] [PubMed] [Google Scholar]
- Bostian K. A., Jayachandran S., Tipper D. J. A glycosylated protoxin in killer yeast: models for its structure and maturation. Cell. 1983 Jan;32(1):169–180. doi: 10.1016/0092-8674(83)90507-x. [DOI] [PubMed] [Google Scholar]
- Bostian K. A., Lemire J. M., Halvorson H. O. Physiological control of repressible acid phosphatase gene transcripts in Saccharomyces cerevisiae. Mol Cell Biol. 1983 May;3(5):839–853. doi: 10.1128/mcb.3.5.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bostian K. A., Sturgeon J. A., Tipper D. J. Encapsidation of yeast killer double-stranded ribonucleic acids: dependence of M on L. J Bacteriol. 1980 Jul;143(1):463–470. doi: 10.1128/jb.143.1.463-470.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botstein D., Falco S. C., Stewart S. E., Brennan M., Scherer S., Stinchcomb D. T., Struhl K., Davis R. W. Sterile host yeasts (SHY): a eukaryotic system of biological containment for recombinant DNA experiments. Gene. 1979 Dec;8(1):17–24. doi: 10.1016/0378-1119(79)90004-0. [DOI] [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Bussey H. Physiology of killer factor in yeast. Adv Microb Physiol. 1981;22:93–122. doi: 10.1016/s0065-2911(08)60326-4. [DOI] [PubMed] [Google Scholar]
- Bussey H., Saville D., Greene D., Tipper D. J., Bostian K. A. Secretion of Saccharomyces cerevisiae killer toxin: processing of the glycosylated precursor. Mol Cell Biol. 1983 Aug;3(8):1362–1370. doi: 10.1128/mcb.3.8.1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bussey H., Sherman D., Somers J. M. Action of yeast killer factor: a resistant mutant with sensitive spheroplasts. J Bacteriol. 1973 Mar;113(3):1193–1197. doi: 10.1128/jb.113.3.1193-1197.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casadaban M. J., Martinez-Arias A., Shapira S. K., Chou J. Beta-galactosidase gene fusions for analyzing gene expression in escherichia coli and yeast. Methods Enzymol. 1983;100:293–308. doi: 10.1016/0076-6879(83)00063-4. [DOI] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Empirical predictions of protein conformation. Annu Rev Biochem. 1978;47:251–276. doi: 10.1146/annurev.bi.47.070178.001343. [DOI] [PubMed] [Google Scholar]
- Fink G. R., Styles C. A. Curing of a killer factor in Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1972 Oct;69(10):2846–2849. doi: 10.1073/pnas.69.10.2846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haguenauer-Tsapis R., Hinnen A. A deletion that includes the signal peptidase cleavage site impairs processing, glycosylation, and secretion of cell surface yeast acid phosphatase. Mol Cell Biol. 1984 Dec;4(12):2668–2675. doi: 10.1128/mcb.4.12.2668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanes S. D., Burn V. E., Sturley S. L., Tipper D. J., Bostian K. A. Expression of a cDNA derived from the yeast killer preprotoxin gene: implications for processing and immunity. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1675–1679. doi: 10.1073/pnas.83.6.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattori M., Sakaki Y. Dideoxy sequencing method using denatured plasmid templates. Anal Biochem. 1986 Feb 1;152(2):232–238. doi: 10.1016/0003-2697(86)90403-3. [DOI] [PubMed] [Google Scholar]
- Hopper J. E., Bostian K. A., Rowe L. B., Tipper D. J. Translation of the L-species dsRNA genome of the killer-associated virus-like particles of Saccharomyces cerevisiae. J Biol Chem. 1977 Dec 25;252(24):9010–9017. [PubMed] [Google Scholar]
- Hutchins K., Bussey H. Cell wall receptor for yeast killer toxin: involvement of (1 leads to 6)-beta-D-glucan. J Bacteriol. 1983 Apr;154(1):161–169. doi: 10.1128/jb.154.1.161-169.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julius D., Brake A., Blair L., Kunisawa R., Thorner J. Isolation of the putative structural gene for the lysine-arginine-cleaving endopeptidase required for processing of yeast prepro-alpha-factor. Cell. 1984 Jul;37(3):1075–1089. doi: 10.1016/0092-8674(84)90442-2. [DOI] [PubMed] [Google Scholar]
- Koren R., LeVitre J., Bostian K. A. Isolation of the positive-acting regulatory gene PHO4 from Saccharomyces cerevisiae. Gene. 1986;41(2-3):271–280. doi: 10.1016/0378-1119(86)90107-1. [DOI] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Lathe R., Kieny M. P., Skory S., Lecocq J. P. Linker tailing: unphosphorylated linker oligonucleotides for joining DNA termini. DNA. 1984;3(2):173–182. doi: 10.1089/dna.1984.3.173. [DOI] [PubMed] [Google Scholar]
- Lolle S., Skipper N., Bussey H., Thomas D. Y. The expression of cDNA clones of yeast M1 double-stranded RNA in yeast confers both killer and immunity phenotypes. EMBO J. 1984 Jun;3(6):1383–1387. doi: 10.1002/j.1460-2075.1984.tb01981.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messing J., Crea R., Seeburg P. H. A system for shotgun DNA sequencing. Nucleic Acids Res. 1981 Jan 24;9(2):309–321. doi: 10.1093/nar/9.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsnes S., Pihl A. Isolation and properties of abrin: a toxic protein inhibiting protein synthesis. Evidence for different biological functions of its two constituent-peptide chains. Eur J Biochem. 1973 May;35(1):179–185. doi: 10.1111/j.1432-1033.1973.tb02823.x. [DOI] [PubMed] [Google Scholar]
- Parent S. A., Fenimore C. M., Bostian K. A. Vector systems for the expression, analysis and cloning of DNA sequences in S. cerevisiae. Yeast. 1985 Dec;1(2):83–138. doi: 10.1002/yea.320010202. [DOI] [PubMed] [Google Scholar]
- Perlman D., Halvorson H. O. The MURFI linker for multiple reading frame insertion of a sense or nonsense codon into DNA. Nucleic Acids Res. 1986 Mar 11;14(5):2139–2155. doi: 10.1093/nar/14.5.2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thill G. P., Kramer R. A., Turner K. J., Bostian K. A. Comparative analysis of the 5'-end regions of two repressible acid phosphatase genes in Saccharomyces cerevisiae. Mol Cell Biol. 1983 Apr;3(4):570–579. doi: 10.1128/mcb.3.4.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tipper D. J., Bostian K. A. Double-stranded ribonucleic acid killer systems in yeasts. Microbiol Rev. 1984 Jun;48(2):125–156. doi: 10.1128/mr.48.2.125-156.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickner R. B., Leibowitz M. J. Two chromosomal genes required for killing expression in killer strains of Saccharomyces cerevisiae. Genetics. 1976 Mar 25;82(3):429–442. doi: 10.1093/genetics/82.3.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoller M. J., Smith M. Oligonucleotide-directed mutagenesis of DNA fragments cloned into M13 vectors. Methods Enzymol. 1983;100:468–500. doi: 10.1016/0076-6879(83)00074-9. [DOI] [PubMed] [Google Scholar]