Abstract
Background
Dizziness and unstable gait with resultant falls are common symptoms among the older adults. Most of studies have focused on statistical analysis regarding single factor related to dizziness and unstable gait. On the other hand, there are very few comprehensive studies using a large number of patients except several review papers.
Methods
We retrospectively analyzed a total of 164 aged patients with dizziness and unstable gait. The patients underwent description of the Japanese version of the Dizziness Handicap Inventory (DHI), measurements of vestibular function, handgrip muscle strength, physical performance, height-adjusted appendicular skeletal muscle mass, and vitamin B1 and B12, a full-night polysomnography study, cognition test and visual test.
Results
Average age was 80.5 ± 6.1 years and ranged from 59 to 91 years. Forty-eight were males and 116 females. Three causative factors, namely vestibular hypofunction, muscle dysfunction and sleep disturbance, were independently and combinedly associated with dizziness and unstable gait in over 93% of the patients. Patients with higher scores defined by these three causative factors had higher scores of DHI. 23% of the patients showed vitamin B1 and/or B12 deficiency, which was highly associated with sarcopenia/frailty. Cognitive and visual impairment were recognized in 4.9% and 5.0%, respectively.
Conclusion
Dizziness and unstable gait were mainly associated with vestibular hypofunction, muscle dysfunction and sleep disturbance. In addition, vitamin B1 and B12 deficiency, and cognitive and visual impairment secondarily contribute to dizziness and unstable gait. Appropriate selection of treatment according to the underlying causes would prevent accidental falls among the older adults.
Keywords: Dizziness, Unstable gait, Older adults, Vestibular hypofunction, Sarcopenia, Sleep apnea, Vitamin B, Cognition
Introduction
The proportion of older adults aged 65 and over in Japan is 29.1% in 2022, the highest among major countries. The trend of increase is expected to continue year by year, reaching 38.4% in 2065. Dizziness (unsteadiness) and unstable gait are common symptoms among the older adults. Unstable gait can be expressed by the changes in the strategies for standing and walking: the stance and gait base is widened, bipedal floor contact is prolonged, step length becomes shorter, the feet are lifted less high during the swing phase, walking becomes slower and the posture becomes stooped [1]. In Europe, the proportion of people visiting medical institutions due to unstable gait is reported to be high, at 30% for those aged 70 and over and 50% for those aged 80 and over [2]. In a survey in the United States, a simple clinical function test showed that 85% of people aged 80 and over had balance disorders [3]. Dizziness and unstable gait are also associated with accidental falls among older adults. The annual incidence of falls among older adults living at home in the community in Japan is 10–25%, and among those living in facilities, it is 10–15%. The incidence of injuries due to falls among older adults people is 54–70%, and the number of cases resulting in death is reported to be four times that of traffic accidents [4].
Causes of vertigo or dizziness in the older adults include benign paroxysmal positional vertigo, Meniere’s disease, orthostatic intolerance, and central vertigo. However, age-related balance disorders are characterized by a slowly progressing persistent sense of unsteadiness, instability in posture and gait, and falls. The deterioration of the balance organs due to age-related vestibular disorder [5] and a decrease in visual information due to age-related decline in vision [6] are well known to contribute to dizziness and unstable gait. The loss in muscle mass in old age is a known main risk for gait disorders and falls. Muscle atrophy combined with impairment of muscle function is known as sarcopenia. The prevalence of sarcopenia in patients with dizziness is higher than that in community-dwelling older adult individuals [7]. Moreover, recent cohort studies [8–10] have shown that subjectively reported poor quality sleep, related to obstructive sleep apnea (OSA), is associated with an increased risk of falls. OSA was demonstrated to impair posture/balance and gait with nocturnal hypoxia. A decrease in brain cognition due to sleep disorders and the associated sleep apnea [11] together with a decrease in the cognitive ability of balance due to age-related changes in the brain [12] result in a very complex pathology of dizziness and unstable gait [2]. Furthermore, recent studies documented that low vitamin B1 and B12 levels are associated with physical function [13, 14].
Most of studies have focused on statistical analysis regarding single factor related to dizziness and unstable gait. On the other hand, there are very few comprehensive studies using a large number of patients except several review papers [15, 16]. Thus, the aim of this study is to comprehensively investigate the possible causes of dizziness and unstable gait in the older adults.
Materials and methods
Subjects
In the cross-sectional study, the patients of older adults were subsequently recruited during their visit to the otorhinolaryngology clinic of the Juntendo Tokyo Koto Geriatric Medical Center between December 1, 2021 and January 31, 2024. The inclusion criteria for the patients were as follows: (1) living in the community, and (2) presence of unsteadiness at standing position and unstable gait for at least 6 months. The exclusion criteria for the patients were as follows: (1) a history with inflammatory ear disorders such as chronic otitis media and bacterial labyrinthine diseases, (2) a history of stroke, (3) presence of neurodegenerative diseases, (4) presence of dementia, (5) presence of blindness, and (6) presence of severe cardiopulmonary diseases. The study in compliance with the principles of the Declaration of Helsinki was approved by the hospital ethics committee. All subjects entered the study after signing an informed consent form.
Dizziness handicap inventory and vestibular function test
The Japanese version of the Dizziness Handicap Inventory (DHI) survey was applied to the patients. DHI is a survey that consists of 25 questions that evaluate the patient’s quality of life emotionally, physically, and functionally. It is used for evaluating the effects of dizziness on quality of life in clinical research and studies [17]. For assessing vestibular function, we used caloric tests and calculated the bithermal maximum peak of slow-phase velocity (SPV) of the nystagmus. Severe vestibular weakness was considered if the sum of the bithermal maximum peak of SPV was < 6 °/S [18]. Moderate vestibular hypofunction was set when the sum of the bithermal maximum peak of SPV was between 6 and 25 °/S [5]. The normal cutoff was defined >25 °/S for bithermal response.
Determination of sarcopenia and frailty
Sarcopenia was defined by handgrip muscle strength, physical performance, height-adjusted appendicular skeletal muscle mass according to the Asian Working Group for Sarcopenia 2019 [19]. Frailty was defined as a clinical syndrome in which three or more of the following criteria were present: un-intentional weight loss, self-reported exhaustion, weakness of handgrip strength, slow walking speed, and low physical activity [20]. The cutoff values for low muscle mass are < 7.0 kg/mg2 in men and < 5.7 kg/mg2 in women by bioelectrical impedance analysis. Low handgrip muscle strength was defined as < 28.0 kg for men and < 18.0 kg for women. Physical performance included the gait speed, the five-time chair stand test, and the short physical performance battery. The low physical performance using 4-m gait speed was defined as < 1.0 m/s, 5-time chair stand test ≥ 12 s, or short physical performance battery score < 9. Sarcopenia is defined as low muscle mass plus low muscle strength or combined low physical performance. Severe sarcopenia is defined as low muscle mass plus low muscle strength plus low physical performance.
Assessment of OSA
All participants underwent a full-night polysomnography study with at least 7 h of recording time (Alice PDx5 Diagnostic System; Philips Respironics, Tokyo, Japan). Sleep staging was scored according to the criteria of the American Academy of Sleep Medicine published in 2018. Apnea was defined as the cessation of airflow for 10 s. Hypopnea was defined as a 30% reduction of airflow lasting 10 s and associated with a 4% decrease in oxyhemoglobin saturation. The number of apneas and hypopneas per hour of sleep was calculated to obtain the apnea-hypopnea index (AHI). The participants were classified into three different groups according to the diagnostic criteria for OSA: severe OSA (AHI ≥ 30) and moderate OSA (15 ≤ AHI ≤ 30), and mild OSA/normal (AHI < 15).
Blood tests, cognitive and visual assessment, and other survey variables
Hematological examination including vitamin B1 and B12, Mini-Mental State Examination (MMSE), and examination of visual acuity were performed. Diagnosis of B1 and B12 deficiency was based on serum B1 and B12 concentrations below 24 ng/ml and 200 pg/mL, respectively. MMSE ranges from 0 to 30 points and patients scoring ≤ 23 considered cognitively impaired [21]. Visual impairment was defined as presenting visual acuity < 6/18 to 3/60 and blindness as < 3/60 [22]. Spirometry, electrocardiogram, and measurement of ankle brachial pressure index were performed in order to exclude the patients with severe cardiopulmonary diseases.
Vestibule-Sarcopenia-Apnea score
Vestibule-Sarcopenia-Apnea (VSA) score was defined as the sum of the scores of vestibule, sarcopenia, and apnea elements followed by (i) vestibule element including bilateral severe hypofunction (score 3), bilateral moderate hypofunction or unilateral severe hypofunction (score 2), unilateral moderate hypofunction (score 1), and no impairment (score 0), (ii) sarcopenia element including severe sarcopenia (score 3), sarcopenia (score 2), frailty (score 1), and no sarcopenia or frailty (score 0) and (iii) apnea element including severe OSA (score 2), moderate OSA (score 1), and mild OSA/normal (score 0).
Statistics
For comparison of sex difference, Pearson’s chi-square test was used. The normality of the data was assessed by the Shapiro-Wilk test. Normally distributed data were expressed as the mean±standard deviation, and were analyzed by one-way analysis of variance (ANOVA) with Bonferroni correction in DHI scores related to VSA scores. Differences were considered to be significant if P < 0.05. Statistical analysis was performed with SPSS version 26 software (IBM Corp., Armonk, New York, USA).
Results
A total of 164 patients with dizziness and unstable gait were recruited. Average age was 80.5 ± 6.1 years and ranged from 59 to 91 years. Forty-eight were men and 116 women; the female/male ratio was 2.41/1. Most of the patients were ranged between 75 and 89 years. The patients between 80 and 84 years were most frequently recognized (Fig. 1). Three major factors of high prevalence were vestibular hypofunction, sarcopenia/frailty, and OSA. The others include vitamin B1/B12 deficiency, cognitive impairment, and visual impairment (Fig. 2). All patients with vitamin B1/B12 deficiency accompany at least one parameter such as vestibular hypofunction, sarcopenia/frailty, and/or OSA. Especially, 61% of patients with vitamin B1/B12 deficiency was associated with sarcopenia/frailty. Cognitive impairment was observed in 8 patients where vestibular hypofunction, sarcopenia/frailty, and OSA were associated in 8, 7, and 4 patients, respectively. Visual impairment was recognized in 8 patients where vestibular hypofunction, sarcopenia/frailty, and OSA were associated in 6, 4, and 4 patients, respectively. One patient with visual impairment had no association with other parameters.
Fig. 1.
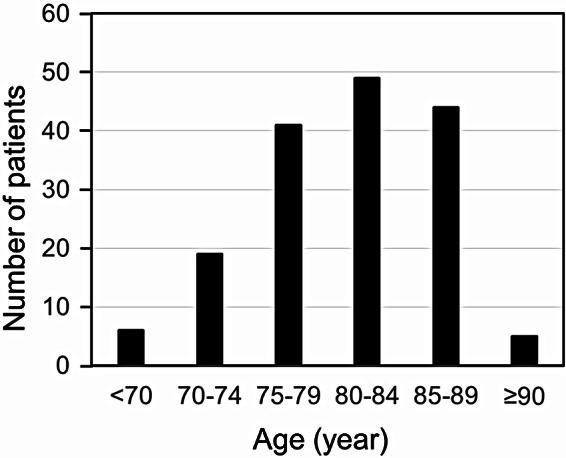
The distribution of the age of 5-year intervals
Fig. 2.
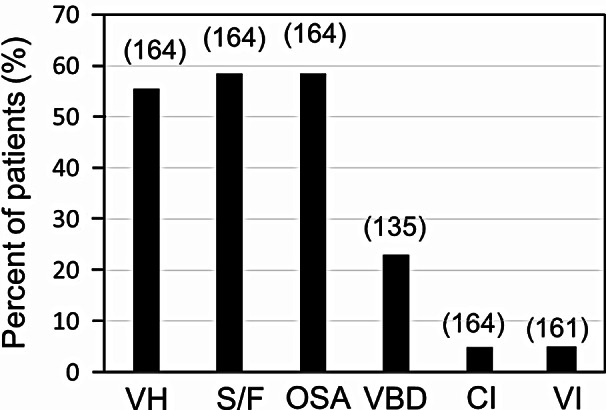
The prevalence of vestibular hypofunction, sarcopenia/frailty, obstructive sleep apnea (severe and moderate), vitamin B1/B12 deficiency, cognitive impairment, and visual impairment. Parentheses: number of patients examined. Abbreviation: VH, vestibular hypofunction; S/F, sarcopenia/frailty; OSA, obstructive sleep apnea (severe and moderate); VBD, vitamin B1/B12 deficiency; CI, cognitive impairment; VI, visual impairment
The prevalence of male and female in vestibular hypofunction, sarcopenia/frailty, and OSA was analyzed. No significant differences were found between the sex of the patients in the prevalence of vestibular hypofunction and sarcopenia/frailty whereas the prevalence of OSA in male was significantly greater than that in female (Table 1).
Table 1.
The prevalence of male and female in vestibular hypofunction, sarcopenia/frailty, and sleep apnea
| Gender | Presence of vestibular hypofunction | Presence of sarcopenia/frailty | Presence of sleep apnea | |
|---|---|---|---|---|
| Male | 67% | 52% | 71% | |
| Female | 51% | 71% | 53% | |
| Pearson`s chi-square test | P = 0.064 | P = 0.281 | P = 0.040 |
The distribution of the prevalence of vestibular hypofunction, sarcopenia/frailty, and OSA was shown in Fig. 3. Thirty-one patients (19%) showed positive findings of all the three parameters. Sixty-eight patients (41%) showed positive for two parameters, namely, 16% positive for vestibular hypofunction and OSA, 13% positive for sarcopenia/frailty and OSA, and 12% positive for vestibular hypofunction and sarcopenia/frailty. Fifty-four patients (33%) show positive for one parameter, namely 14% positive for sarcopenia/frailty, 11% positive for OSA, and 8% positive for vestibular hypofunction. Eleven patients (7%) showed no apparent disturbance in three parameters; 4 with low muscle mass in the lower limb, 1 patient with visual impairment, 2 with mild vestibular hypofunction, 2 with mild OSA, and 2 with unknown causes. In other words, 153 out of 164 patients (> 93%) can explain that these three parameters are responsible for dizziness and unstable gait.
Fig. 3.
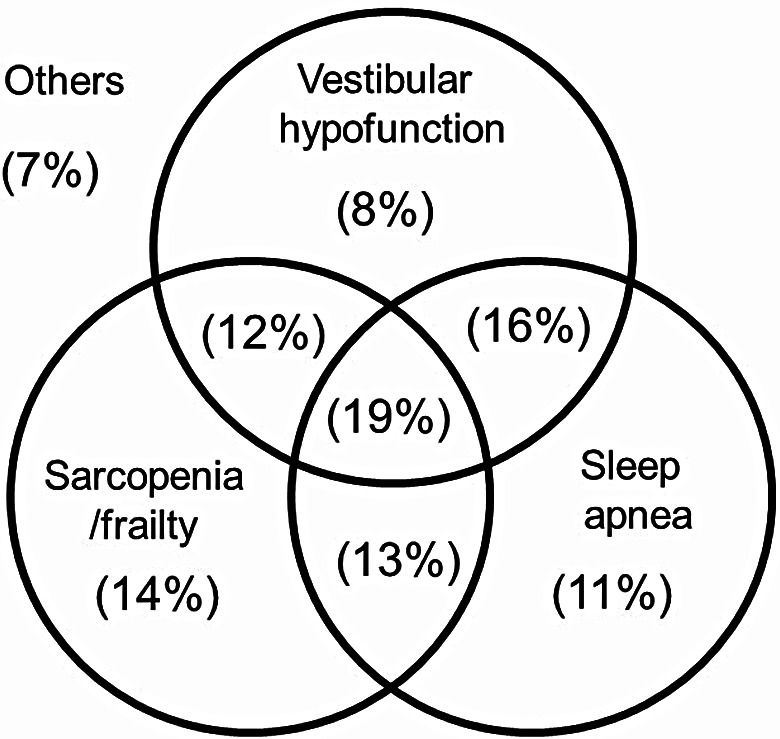
The distribution of the prevalence of vestibular hypofunction, sarcopenia/frailty, and sleep apnea
Bilateral mild hypofunction was most frequently observed, followed by bilateral severe hypofunction, unilateral severe hypofunction, and unilateral mild hypofunction except for bilateral normal function. Frailty was most frequently observed, followed by severe sarcopenia, and sarcopenia except for normal muscular property. Severe apnea was most frequently seen, followed by mild/normal and moderate apnea (Table 2).
Table 2.
The distribution of the detailed types of vestibular hypofunction, sarcopenia/frailty, and sleep apnea
| Classification of parameters | Number of patients (%) |
|---|---|
| Bilateral severe hypofunction | 25 (15) |
| Bilateral moderate hypofunction | 47 (29) |
| Unilateral severe hypofunction | 13 (8) |
| Unilateral moderate hypofunction | 6 (4) |
| Normal | 73 (44) |
| Severe sarcopenia | 15 (9) |
| Sarcopenia | 11 (7) |
| Frailty | 70 (43) |
| Normal | 68 (41) |
| Severe apnea | 67 (41) |
| Moderate apnea | 47 (29) |
| Mild apnea or normal | 48 (29) |
| Unkown | 2 (1) |
The VSA scores took a normal distribution with a peak at score of 3 shown in Fig. 4. The relationship between DHI scores and VSA scores was shown in Fig. 5. DHI scores were greater in the patients with the VSA scores of 6–8 (P = 0.038) and of 3–5 (P = 0.043) than those with the VSA scores of 0–2. There was no significant difference between those of the VSA scores of 3–5 and of 6–8.
Fig. 4.
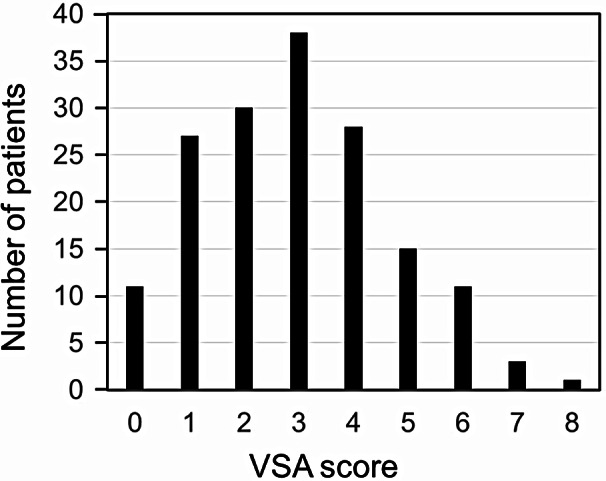
The distribution of the number of patients in the vestibule-sarcopenia-apnea score. Abbreviation: VSA, Vestibule-Sarcopenia-Apnea
Fig. 5.
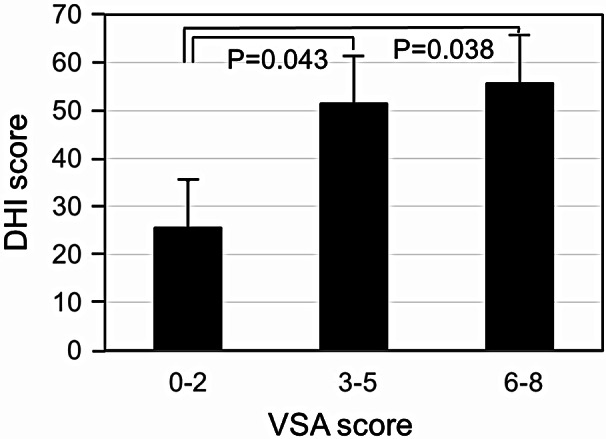
The relationship between DHI scores and VSA scores. Abbreviations: DHI, Dizziness Handicap Inventory; VSA, Vestibule-Sarcopenia-Apnea
Discussion
The present cross-sectional study was conducted to investigate the major causes of dizziness and unstable gait in the aged populations, revealing that (i) dizziness and unstable gait were independently and combinedly associated with vestibular hypofunction, muscle dysfunction and sleep disturbance, and these three causative factors explain dizziness and unstable gait in over 93% of the patients; (ii) a significant difference was found between the sex of the patients in the prevalence of OSA (severe/moderate), but not in vestibular hypofunction or sarcopenia/frailty, (iii) patients with higher scores defined by three major causative factors had higher scores of DHI, (iv) 23% of the patients showed vitamin B1 and/or B12 deficiency, which was highly associated with sarcopenia/frailty. This study would make a strong impact on therapeutic strategy and modalities of accidental falls with unknown origins in numerous older adults.
The present study showed a higher prevalence of dizziness and unstable gait in females than males. A possible underlying reason for the higher prevalence in female is related to the fact that the perception of disability is much higher in female than in male. This may be explained by the differences that persist between sexes in terms of work activities and responsibility by housework [23].
Age-related vestibular dysfunction, presbyvestibulopathy, was proposed by the Barany Society in 2019 as a chronic vestibular syndrome characterized by postural instability, gait disorders, and repeated falls as a result of decreased bilateral vestibular function due to aging [5]. The diagnostic criteria are to meet all four of the following four criteria: (i) chronically persistent vestibular symptoms, (ii) bilateral vestibular dysfunction determined by a balance function test, (iii) age 60 or older, and (iv) the condition cannot be explained by another disease or disorder. Chronic unilateral vestibular dysfunction caused by sudden hearing loss, Meniere’s disease, vestibular neuritis, etc. would show persistent unsteadiness and instability in maintaining posture. The symptoms induced by chronic unilateral vestibular dysfunction are thought to be due to vestibular decompensation, psychological factors, and dependency on visual information [24]. There is strong evidence supporting vestibular physical therapy for reducing symptoms, improving gaze and postural stability, and improving function in individuals with vestibular hypofunction. Vestibular rehabilitation is recommended to adults with unilateral and bilateral vestibular hypofunction who present with impairments, activity limitations, and participation restriction related to the vestibular deficit [25].
Decreased muscle mass in the older adults is known to be a risk factor for gait disorders and falls. Sarcopenia is defined as “a decrease in skeletal muscle mass with aging” and is diagnosed when there is a decrease in limb skeletal muscle mass as well as a decrease in physical function or muscle strength. Specifically, sarcopenia is determined by three indices: muscle strength, function, and volume. Frailty is a state in which various physical and mental functions gradually decline due to aging or disease, making the body vulnerable to mental and physical stress. Frailty is diagnosed when three or more of the following criteria are met: (i) weight loss, (ii) fatigue, (iii) decreased walking speed, (iv) decreased grip strength, and (v) decreased physical activity. Muscle (speed) training, preferably in combination with a sufficiently high protein intake (1.2–1.5 g/kg body weight/day) is eminently suitable for regaining muscle strength in older persons [26]. A systematic review of Japanese community-dwelling older adults documented that the pooled prevalence rates of sarcopenia were 9.9% among men and 10.1% among women [27]. Age-stratified meta-analyses of four studies showed an overall pooled prevalence of frailty among Japanese community-dwelling older people of 7.4% [28]. The cross-sectional study included 331 Japanese community-dwelling adults in the older adults showed that the prevalence of sarcopenia/frailty was 11.8% [29]. Although this study lacks normal control group, the prevalence of sarcopenia/frailty in this study over 50% is apparently higher than the historical data of the older adults, which suggests a strong relationship between dizziness/unstable gait and sarcopenia/frailty.
Recent studies have gradually revealed that balance disorders, gait disorders, and falls are associated with sleep disorders. Several studies on OSA and insomnia demonstrate evidence of an increased risk for falls [8–10]. It has been reported that treatment of OSA with continuous positive airway pressure improves gait disorders and reduces episodes of falls [30, 31]. In OSA, it is thought that balance and gait disorders occur due to daytime somnolence, anoxia, and hypercapnia causing decreased vestibulo-ocular reflex, decreased vision due to optic nerve thinning and glaucoma, decreased cognitive ability, and decreased lower limb muscle strength [11]. It is well known that the prevalence of OSA is two- to three-fold higher in males than in females [32]. The prevalence of self-reported OSA was estimated to be 4 to 9% in aged females and 12 to 24% in aged males in western countries and Japan [33–35]. Similarly, the present study showed a significant increase of prevalence in men as compared with female. Furthermore, the prevalence of this study is apparently higher than that of general populations of the older adults in both genders, suggesting that OSA is a causative and risk factor for dizziness and unstable gait.
The older adults to very older adults are prone to vitamin deficiency due to poor diet. Vitamin B1 has a small internal storage and a short half-life, so continuous intake is necessary. Peripheral neuropathy caused by vitamin B1 deficiency shows symmetric motor and sensory polyneuropathy starting from the distal lower limbs, and often progresses over a period of weeks. Vitamin B12 deficiency neuropathy also shows slow-onset sensory and motor polyneuropathy. The reduced motor function of the lower limbs caused by vitamin B1 and B12 deficiency can cause unsteadiness and gait disorders [36, 37]. Interestingly, several reports postulated that vitamin B1 and B12 are related to sarcopenia and frailty syndrome [13, 14, 38]. Vitamin B12 might cause the decrease in muscle mass by reducing DNA synthesis of human body muscle, reducing the proliferation and differentiation of stem cells necessary for skeletal muscle regeneration, causing nerve demyelination along axons to muscle resulting in axonal deterioration [13]. In the present study, vitamin B1/B12 deficiency is associated with at least one of three major causative factors, implying that vitamin B1/12 deficiency have a secondary effect on dizziness and unstable gait by modulating three major causative factors. Further studies are required to clarify the underlying mechanism of vitamin B1/B12 deficiency regarding dizziness and unstable gait.
Although many people with vestibular disorders experience cognition problems [12], there are no studies regarding patients with dizziness and unstable gait to assess general cognition function using standardized examination. The present study revealed that 4.9% of the older adult patients with dizziness and unstable gait showed cognitive impairment evaluated by MMSE. All patients with cognitive impairment were associated with three major causative factors for dizziness and unstable gait, suggesting that cognitive impairment may play an additional or secondary role in dizziness and instable gait induced by three major causative factors. Nevertheless, cognitive impairment is known to be related to each of three major causative factors [12, 39, 40]. Postural control is dependent upon the integration of information from the proprioceptive, vestibular, and visual sensory systems. The visual element of balance control is influenced by central and peripheral vision as well as eye movements and postural stability has been shown to be reduced in patients with age-related eye disease and eye movement disorders. A recent systematic review documented that five studies found an independent link between dizziness and vison, five found weak association, and three found no association. The overall evidence suggests that dizziness is linked with poor vision [6]. In present finding, 5.0% of 161 patients had visual impairment, and most of the patients with visual impairment except one were associated with three major causative factors. Although visual impairment is suggested to secondarily influence dizziness and unstable gait, the possibility that visual impairment directly causes dizziness and unstable gait is not neglected. Thus, a complex combination of vestibular, muscular, and sleep disturbance together with cognitive and visual impairment may exacerbate dizziness and unstable gait.
The results of our study have some limitations to be considered. First, this was a cross-sectional study. However, the results of relatively high number of patients are meaningful to clarify the characteristic features and the underlying mechanism of dizziness and unstable gait in the older adults. Further prospective studies or randomized controlled trials are needed to confirm our findings. Second, vestibular battery used in this study seems to be weak since caloric tests evaluate the lateral semicircular canal and low frequency vestibulo-ocular reflex responses. The video-head impulse test, which can evaluate high frequency vestibulo-ocular reflex responses in real-life situation, and functional trials such as the Berg Balance Scale could provide more detailed information regarding the vestibular and balance status of the older adults. Third, the present study did not include therapeutic results such as vestibular rehabilitation, diet intake and supplement, and continuous positive airway pressure. Improving symptoms by selecting the appropriate treatment for the individual pathology may lead to a correct diagnosis and contribute to a better understanding of the underlying mechanism.
Conclusion
The results of this cross-sectional study, despite several limitations, are thought to imply the independent and combined association between dizziness and unstable gait in the older adults and three major causative factors such as vestibular hypofunction, muscle dysfunction and sleep disturbance. In addition, dizziness and unstable gait are considered to be brought about secondarily by vitamin B1/B12 deficiency, and cognitive and visual impairment. Appropriate selection of treatment according to the underlying causes would prevent accidental falls accompanied by dizziness and unstable gait among the older adults.
Acknowledgements
The authors are grateful to the members participating in the study program in the Juntendo Tokyo Koto Geriatric Medical Center.
Abbreviations
- DHI
Dizziness Handicap Inventory
- SPV
Slow-Phase Velocity
- AHI
Apnea-Hypopnea Index
- OSA
Obstructive Sleep Apnea
- MMSE
Mini-Mental State Examination
- VSA
Vestibule-Sarcopenia-Apnea
Author contributions
KI designed the study, performed the data analysis, and wrote the paper. KT and ST supported data analysis design. TT, MS, and KO supported the research and revised the manuscript.
Data availability
Data is provided within the manuscript or supplementary information files. The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
The study in compliance with the principles of the Declaration of Helsinki was approved by the Juntendo Tokyo Koto Geriatric Medical Center ethics committee. Clinical trial number: E24-0281. All subjects entered the study after signing an informed consent form.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Pirker W, Katzenschlager R. Gait disorders in adults and the older adults: A clinical guide. Wien Klin Wochenschr. 2017;129(3–4):81–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jahn K, Kressig RW, Bridenbaugh SA, Brandt T, Schniepp R. Dizziness and Unstable Gait in Old Age: Etiology, Diagnosis and Treatment. Dtsch Arztebl Int. 2015;112(23):387–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Agrawal Y, Carey JP, Della Santina CC, Schubert MC, Minor LB. Disorders of balance and vestibular function in US adults: data from the National Health and Nutrition Examination Survey, 2001–2004. Arch Intern Med. 2009;169(10):938–44. [DOI] [PubMed] [Google Scholar]
- 4.Hagino H. Epidemiology and Evidence of Prevention for Falls. Japanese J Rehabilitation Med. 2018;55(11):898–904. [Google Scholar]
- 5.Agrawal Y, Van de Berg R, Wuyts F, Walther L, Magnusson M, Oh E, et al. Presbyvestibulopathy: Diagnostic criteria Consensus document of the classification committee of the Barany Society. J Vestib Res. 2019;29(4):161–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Armstrong D, Charlesworth E, Alderson AJ, Elliott DB. Is there a link between dizziness and vision? A systematic review. Ophthalmic Physiol Opt. 2016;36(4):477–86. [DOI] [PubMed] [Google Scholar]
- 7.Kamo T, Ogihara H, Tanaka R, Kato T, Azami M, Tsunoda R, et al. Prevalence and Risk Factors of Sarcopenia in Patients with Dizziness. Otol Neurotol. 2022;43(9):e1024–8. [DOI] [PubMed] [Google Scholar]
- 8.Stone KL, Blackwell TL, Ancoli-Israel S, Cauley JA, Redline S, Marshall LM, et al. Sleep disturbances and risk of falls in older community-dwelling men: the outcomes of Sleep Disorders in Older Men (MrOS Sleep) Study. J Am Geriatr Soc. 2014;62(2):299–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Degache F, Goy Y, Vat S, Haba Rubio J, Contal O, Heinzer R. Sleep-disordered breathing and daytime postural stability. Thorax. 2016;71(6):543–8. [DOI] [PubMed] [Google Scholar]
- 10.Micarelli A, Liguori C, Viziano A, Izzi F, Placidi F, Alessandrini M. Integrating postural and vestibular dimensions to depict impairment in moderate-to-severe obstructive sleep apnea syndrome patients. J Sleep Res. 2017;26(4):487–94. [DOI] [PubMed] [Google Scholar]
- 11.Stevens D, Jackson B, Carberry J, McLoughlin J, Barr C, Mukherjee S, et al. The Impact of Obstructive Sleep Apnea on Balance, Gait, and Falls Risk: A Narrative Review of the Literature. J Gerontol Biol Sci Med Sci. 2020;75(12):2450–60. [DOI] [PubMed] [Google Scholar]
- 12.Smith LJ, Wilkinson D, Bodani M, Surenthiran SS. Cognition in vestibular disorders: state of the field, challenges, and priorities for the future. Front Neurol. 2024;15:1159174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chae SA, Kim HS, Lee JH, Yun DH, Chon J, Yoo MC et al. Impact of Vitamin B12 Insufficiency on Sarcopenia in Community-Dwelling Older Korean Adults. Int J Environ Res Public Health. 2021;18(23). [DOI] [PMC free article] [PubMed]
- 14.Yang S, Dong Z, Zhao J, Yuan L, Xiao Y, Luo X, et al. Association of vitamins B1 and B2 intake with early-onset sarcopenia in the general adult population of the US: a cross-sectional study of NHANES data from 2011 to 2018. Front Nutr. 2024;11:1369331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dominguez RO, Bronstein AM. Assessment of unexplained falls and gait unsteadiness: the impact of age. Otolaryngol Clin North Am. 2000;33(3):637–57. [DOI] [PubMed] [Google Scholar]
- 16.Jahn K. The Aging Vestibular System: Dizziness and Imbalance in the Older adults. Adv Otorhinolaryngol. 2019;82:143–9. [DOI] [PubMed] [Google Scholar]
- 17.Jacobson GP, Newman CW. The development of the Dizziness Handicap Inventory. Arch Otolaryngol Head Neck Surg. 1990;116(4):424–7. [DOI] [PubMed] [Google Scholar]
- 18.Strupp M, Kim JS, Murofushi T, Straumann D, Jen JC, Rosengren SM, et al. Bilateral vestibulopathy: Diagnostic criteria Consensus document of the Classification Committee of the Barany Society. J Vestib Res. 2017;27(4):177–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen LK, Woo J, Assantachai P, Auyeung TW, Chou MY, Iijima K, et al. Asian Working Group for Sarcopenia: 2019 Consensus Update on Sarcopenia Diagnosis and Treatment. J Am Med Dir Assoc. 2020;21(3):300–e72. [DOI] [PubMed] [Google Scholar]
- 20.Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol Biol Sci Med Sci. 2001;56(3):M146–56. [DOI] [PubMed] [Google Scholar]
- 21.Tsoi KK, Chan JY, Hirai HW, Wong SY, Kwok TC. Cognitive Tests to Detect Dementia: A Systematic Review and Meta-analysis. JAMA Intern Med. 2015;175(9):1450–8. [DOI] [PubMed] [Google Scholar]
- 22.Blindness GBD, Vision Impairment C, Vision Loss Expert Group of the Global Burden of Disease S. Trends in prevalence of blindness and distance and near vision impairment over 30 years: an analysis for the Global Burden of Disease Study. Lancet Glob Health. 2021;9(2):e130–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Soto-Varela A, Rossi-Izquierdo M, Del-Rio-Valeiras M, Vaamonde-Sanchez-Andrade I, Faraldo-Garcia A, Lirola-Delgado A, et al. Presbyvestibulopathy, Comorbidities, and Perception of Disability: A Cross-Sectional Study. Front Neurol. 2020;11:582038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Panichi R, Faralli M, Bruni R, Kiriakarely A, Occhigrossi C, Ferraresi A, et al. Asymmetric vestibular stimulation reveals persistent disruption of motion perception in unilateral vestibular lesions. J Neurophysiol. 2017;118(5):2819–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hall CD, Herdman SJ, Whitney SL, Anson ER, Carender WJ, Hoppes CW, et al. Vestibular Rehabilitation for Peripheral Vestibular Hypofunction: An Updated Clinical Practice Guideline From the Academy of Neurologic Physical Therapy of the American Physical Therapy Association. J Neurol Phys Ther. 2022;46(2):118–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Deutz NE, Bauer JM, Barazzoni R, Biolo G, Boirie Y, Bosy-Westphal A, et al. Protein intake and exercise for optimal muscle function with aging: recommendations from the ESPEN Expert Group. Clin Nutr. 2014;33(6):929–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Makizako H, Nakai Y, Tomioka K, Taniguchi Y. Prevalence of sarcopenia defined using the Asia Working Group for Sarcopenia criteria in Japanese community-dwelling older adults: A systematic review and meta-analysis. Phys Ther Res. 2019;22(2):53–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kojima G, Iliffe S, Taniguchi Y, Shimada H, Rakugi H, Walters K. Prevalence of frailty in Japan: A systematic review and meta-analysis. J Epidemiol. 2017;27(8):347–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mori H, Tokuda Y. Differences and overlap between sarcopenia and physical frailty in older community-dwelling Japanese. Asia Pac J Clin Nutr. 2019;28(1):157–65. [DOI] [PubMed] [Google Scholar]
- 30.Allali G, Perrig S, Cleusix M, Herrmann FR, Adler D, Gex G, et al. Gait abnormalities in obstructive sleep apnea and impact of continuous positive airway pressure. Respir Physiol Neurobiol. 2014;201:31–3. [DOI] [PubMed] [Google Scholar]
- 31.Onen F, Higgins S, Onen SH. Falling-asleep-related injured falls in the older adults. J Am Med Dir Assoc. 2009;10(3):207–10. [DOI] [PubMed] [Google Scholar]
- 32.Lévy P, Kohler M, McNicholas WT, Barbé F, McEvoy RD, Somers VK, et al. Obstructive sleep apnoea syndrome. Nat Rev Dis Primers. 2015;1:15015. [DOI] [PubMed] [Google Scholar]
- 33.Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol. 2013;177(9):1006–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huang T, Lin BM, Markt SC, Stampfer MJ, Laden F, Hu FB et al. Sex differences in the associations of obstructive sleep apnoea with epidemiological factors. Eur Respir J. 2018;51(3). [DOI] [PMC free article] [PubMed]
- 35.Matsumoto T, Murase K, Tabara Y, Gozal D, Smith D, Minami T et al. Impact of sleep characteristics and obesity on diabetes and hypertension across genders and menopausal status: the Nagahama study. Sleep. 2018;41(7). [DOI] [PubMed]
- 36.Matteini AM, Walston JD, Fallin MD, Bandeen-Roche K, Kao WH, Semba RD, et al. Markers of B-vitamin deficiency and frailty in older women. J Nutr Health Aging. 2008;12(5):303–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Oberlin BS, Tangney CC, Gustashaw KA, Rasmussen HE. Vitamin B12 deficiency in relation to functional disabilities. Nutrients. 2013;5(11):4462–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pyrgioti EE, Karakousis ND. B12 levels and frailty syndrome. J Frailty Sarcopenia Falls. 2022;7(1):32–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dzierzewski JM, Dautovich N, Ravyts S. Sleep and Cognition in Older Adults. Sleep Med Clin. 2018;13(1):93–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hu Y, Peng W, Ren R, Wang Y, Wang G. Sarcopenia and mild cognitive impairment among older adults adults: The first longitudinal evidence from CHARLS. J Cachexia Sarcopenia Muscle. 2022;13(6):2944–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data is provided within the manuscript or supplementary information files. The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.


