Abstract
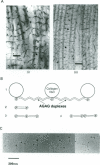
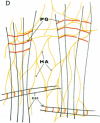
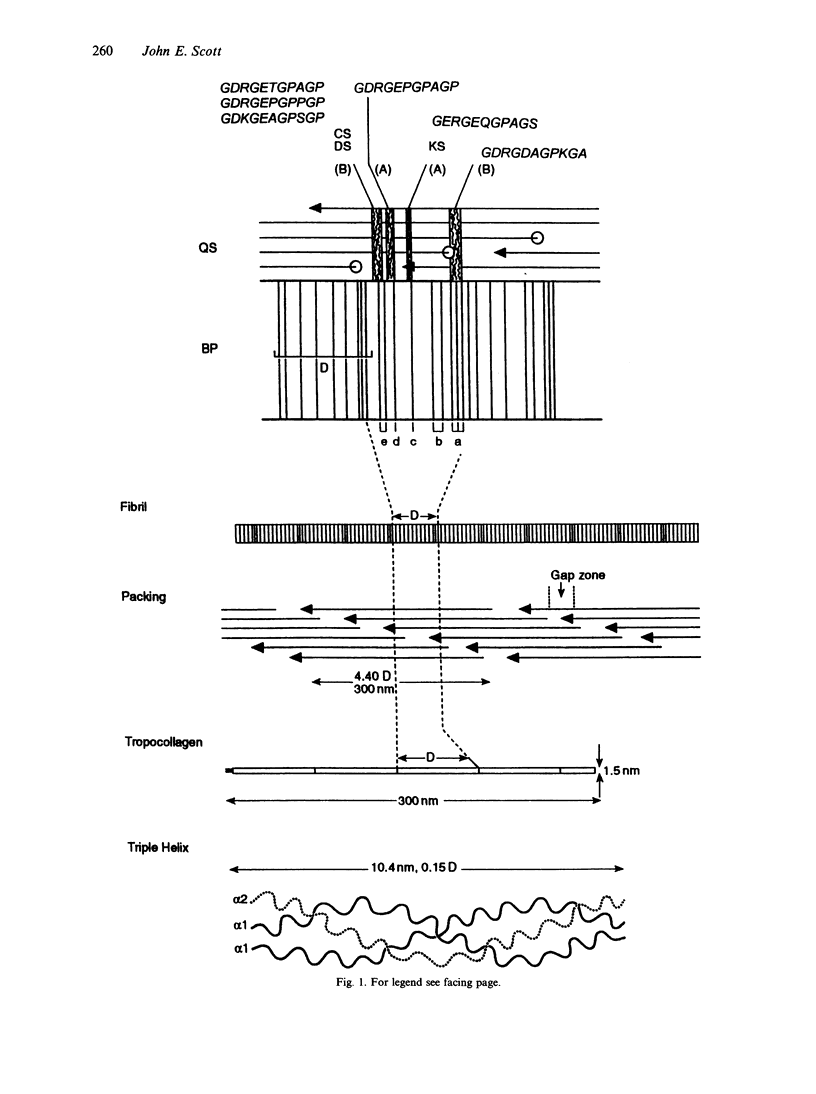
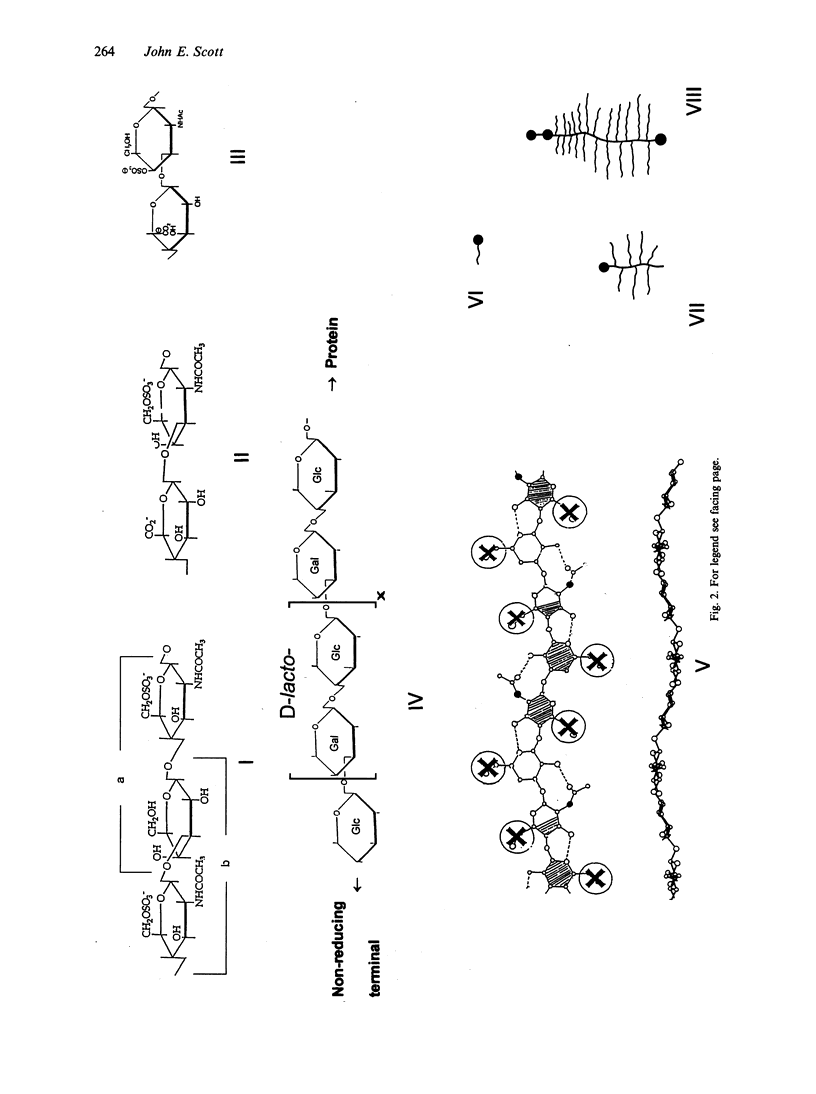
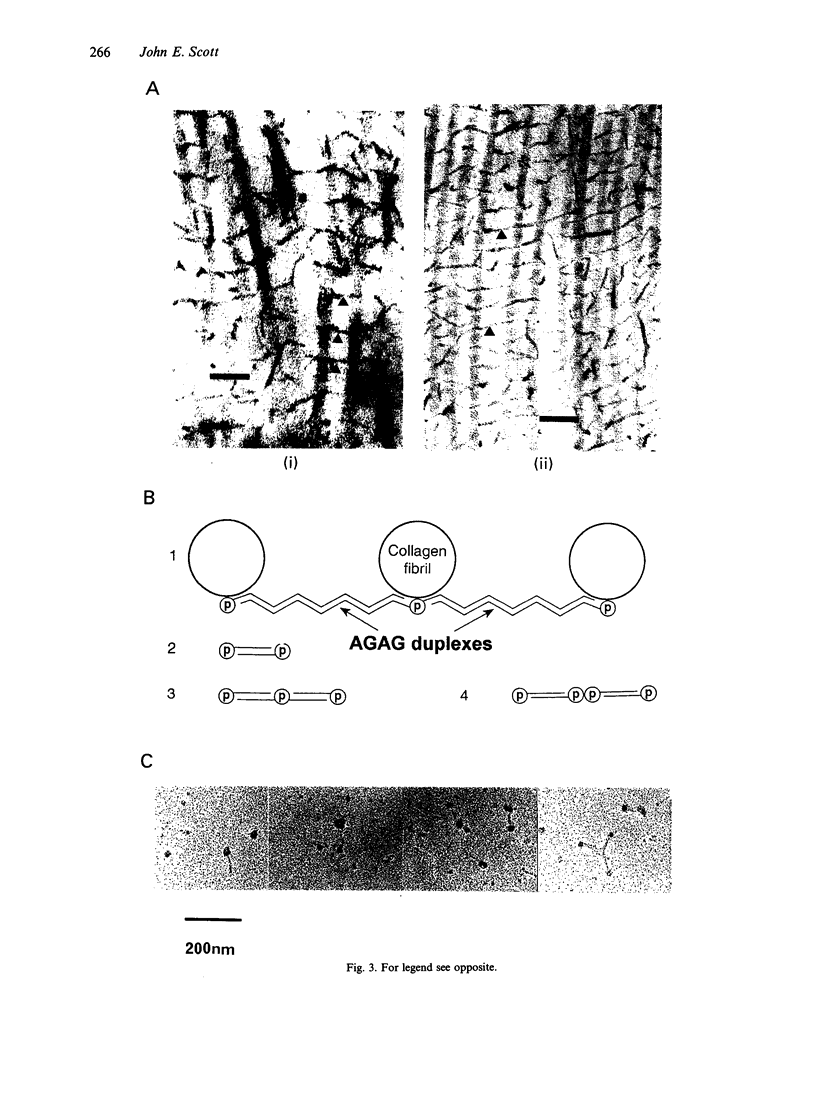
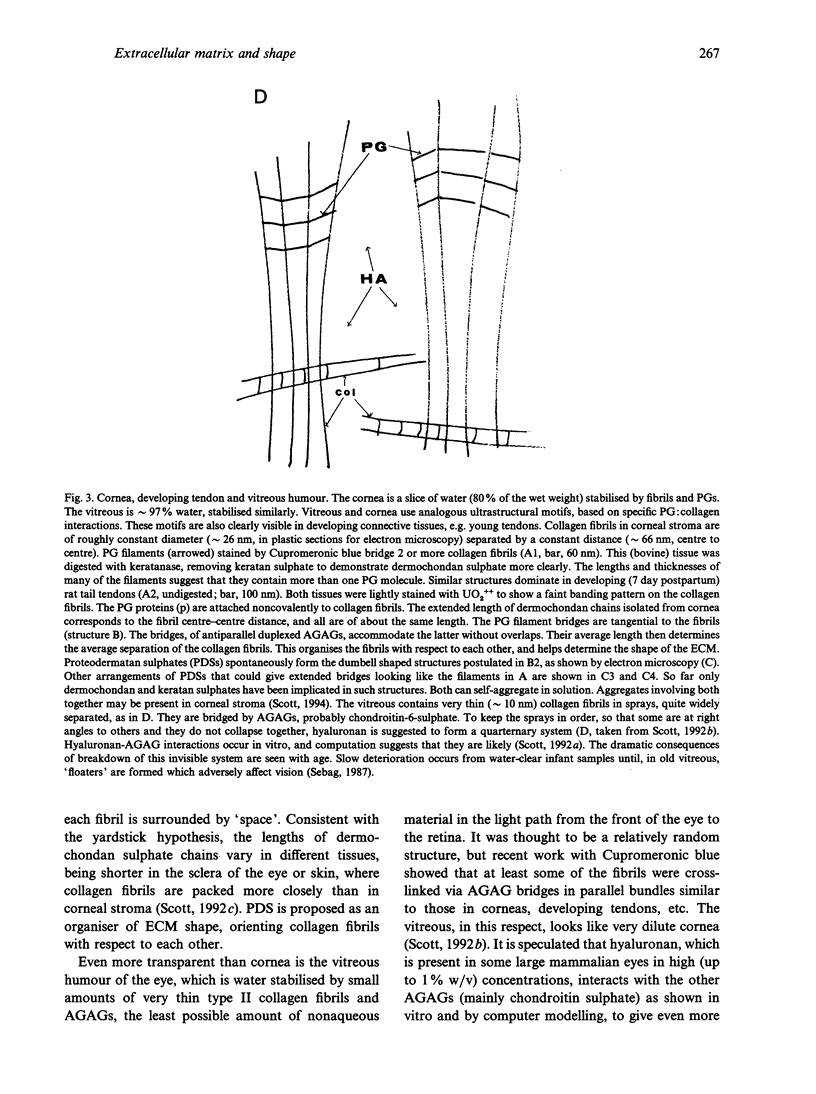
Connective tissue function is defined as the formation and maintenance of shape, without which centralised physiologies (circulatory, digestive or nervous) could not have evolved. Two elements, inextensible (collagenous) fibrils and compression-resistant interfibrillar soluble polymers (proteoglycans), cope with all usual stresses. Relationships between the two are highly specific, as demonstrated by electron histochemistry based on Cupromeronic blue and critical electrolyte concentration (CEC) methodologies. Recent ideas on (1) the protofibrillar or modular structure of collagen fibrils, (2) the nature of specific binding sites for proteoglycans on fibrils, and (3) fundamental similarities in secondary and tertiary structures of the glycosaminoglycans (hyaluronan, chondroitin, keratan and dermatan sulphates) are described. They have greatly illuminated the study of extracellular matrix structure and function in normal, pathological (osteogenesis imperfecta) and ageing tissues. The small proteoglycans are proposed to be tissue organisers, orienting and ordering the collagen fibrils--thus shaping the tissue at a molecular and ultimately macro level. These interfibrillar structures are based on their bifunctional character, the protein parts binding to collagen fibrils at specific sites and the glycosaminoglycans duplexing and aggregating to hold the proteins and hence the collagen fibrils at defined distances from each other, rather like yardsticks. Examples of the way these functions work in specific tissues are drawn from the cornea and vitreous humour of the eye and developing tendon.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chang N. S., Boackle R. J., Armand G. Hyaluronic acid-complement interactions--I. Reversible heat-induced anticomplementary activity. Mol Immunol. 1985 Apr;22(4):391–397. doi: 10.1016/0161-5890(85)90123-3. [DOI] [PubMed] [Google Scholar]
- Erlinger R., Welsch U., Scott J. E. Ultrastructural and biochemical observations on proteoglycans and collagen in the mutable connective tissue of the feather star Antedon bifida (Echinodermata, Crinoidea). J Anat. 1993 Aug;183(Pt 1):1–11. [PMC free article] [PubMed] [Google Scholar]
- Scott J. E., Bosworth T. R., Cribb A. M., Gressner A. M. The chemical morphology of extracellular matrix in experimental rat liver fibrosis resembles that of normal developing connective tissue. Virchows Arch. 1994;424(1):89–98. doi: 10.1007/BF00197398. [DOI] [PubMed] [Google Scholar]
- Scott J. E., Cummings C., Brass A., Chen Y. Secondary and tertiary structures of hyaluronan in aqueous solution, investigated by rotary shadowing-electron microscopy and computer simulation. Hyaluronan is a very efficient network-forming polymer. Biochem J. 1991 Mar 15;274(Pt 3):699–705. doi: 10.1042/bj2740699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott J. E., Glanville R. W. Homologous sequences in fibrillar collagens may be proteoglycan binding sites. Biochem Soc Trans. 1993 May;21(2):123S–123S. doi: 10.1042/bst021123s. [DOI] [PubMed] [Google Scholar]
- Scott J. E., Haigh M., Nusgens B., Lapière C. M. Proteoglycan: collagen interactions in dermatosparactic skin and tendon. An electron histochemical study using cupromeronic blue in a critical electrolyte concentration method. Matrix. 1989;9(6):437–442. doi: 10.1016/s0934-8832(11)80012-0. [DOI] [PubMed] [Google Scholar]
- Scott J. E. Morphometry of cupromeronic blue-stained proteoglycan molecules in animal corneas, versus that of purified proteoglycans stained in vitro, implies that tertiary structures contribute to corneal ultrastructure. J Anat. 1992 Feb;180(Pt 1):155–164. [PMC free article] [PubMed] [Google Scholar]
- Scott J. E., Orford C. R. Dermatan sulphate-rich proteoglycan associates with rat tail-tendon collagen at the d band in the gap region. Biochem J. 1981 Jul 1;197(1):213–216. doi: 10.1042/bj1970213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott J. E., Parry D. A. Control of collagen fibril diameters in tissues. Int J Biol Macromol. 1992 Oct;14(5):292–293. doi: 10.1016/s0141-8130(05)80043-1. [DOI] [PubMed] [Google Scholar]
- Scott J. E. Proteoglycan-fibrillar collagen interactions. Biochem J. 1988 Jun 1;252(2):313–323. doi: 10.1042/bj2520313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott J. E. Proteoglycan:collagen interactions and subfibrillar structure in collagen fibrils. Implications in the development and ageing of connective tissues. J Anat. 1990 Apr;169:23–35. [PMC free article] [PubMed] [Google Scholar]
- Scott J. E. Supramolecular organization of extracellular matrix glycosaminoglycans, in vitro and in the tissues. FASEB J. 1992 Jun;6(9):2639–2645. [PubMed] [Google Scholar]
- Scott J. E. The nomenclature of glycosaminoglycans and proteoglycans. Glycoconj J. 1993 Dec;10(6):419–421. doi: 10.1007/BF00737960. [DOI] [PubMed] [Google Scholar]