Abstract
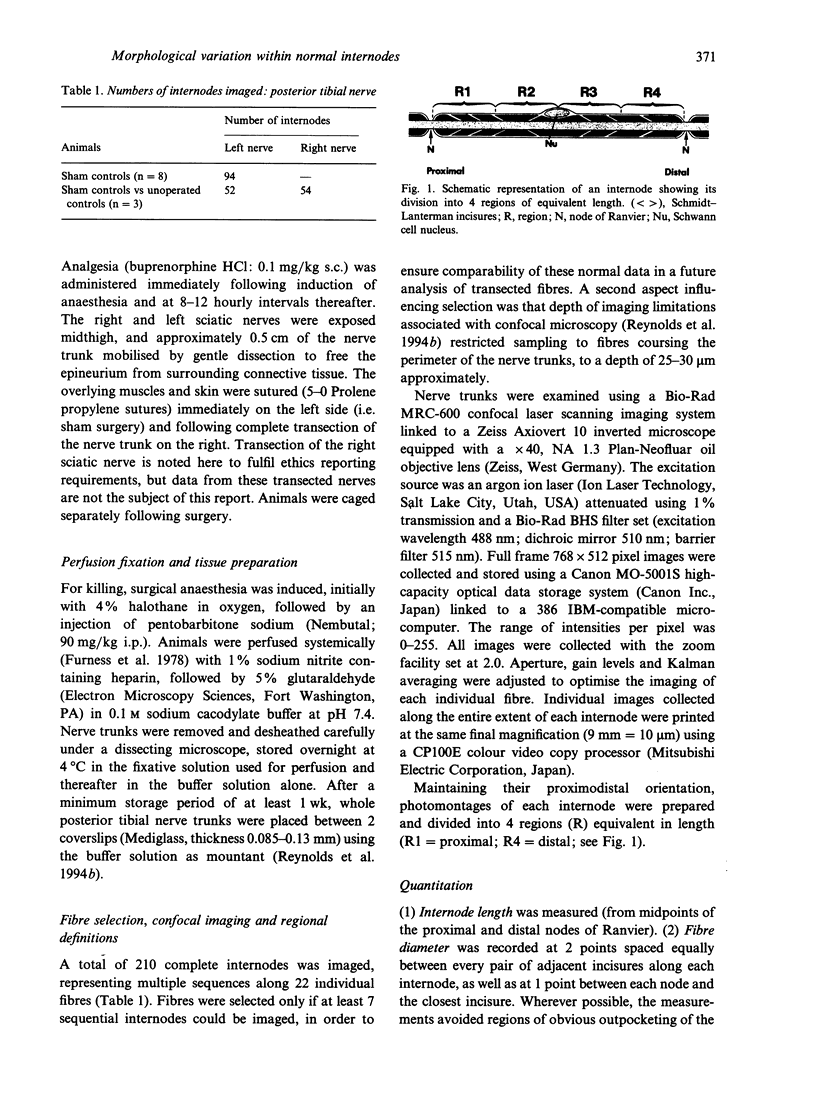
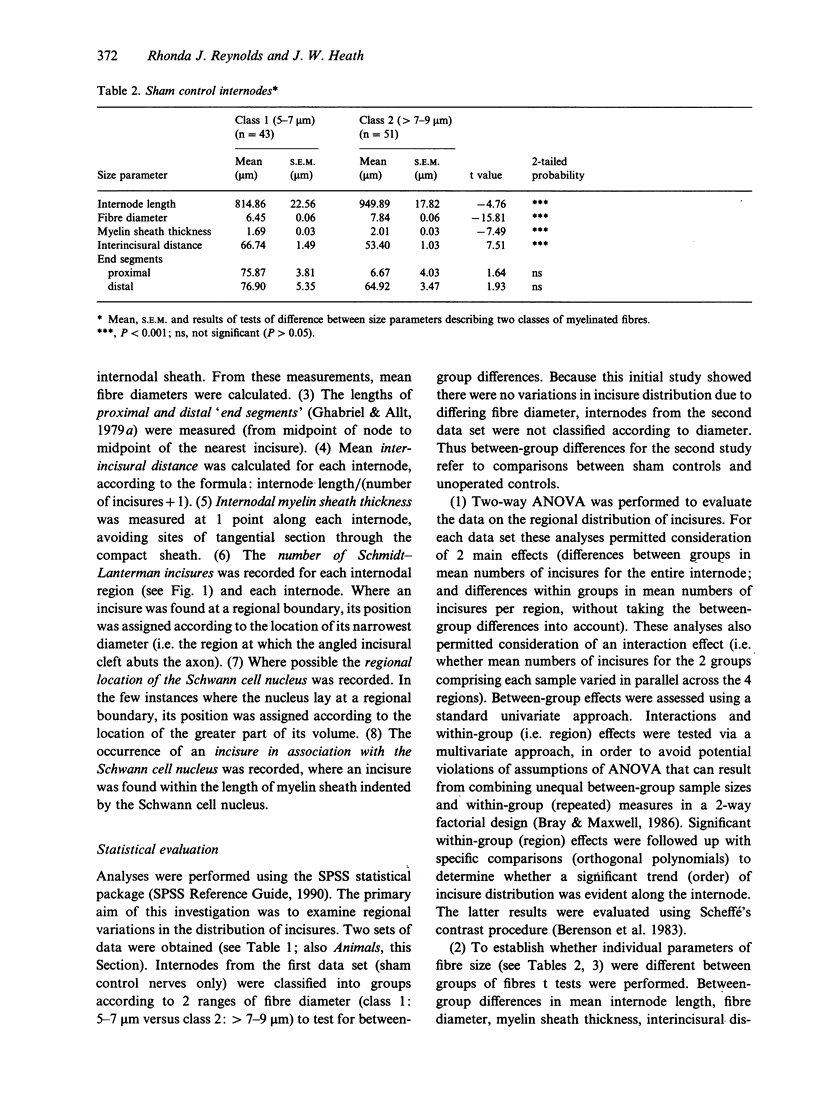
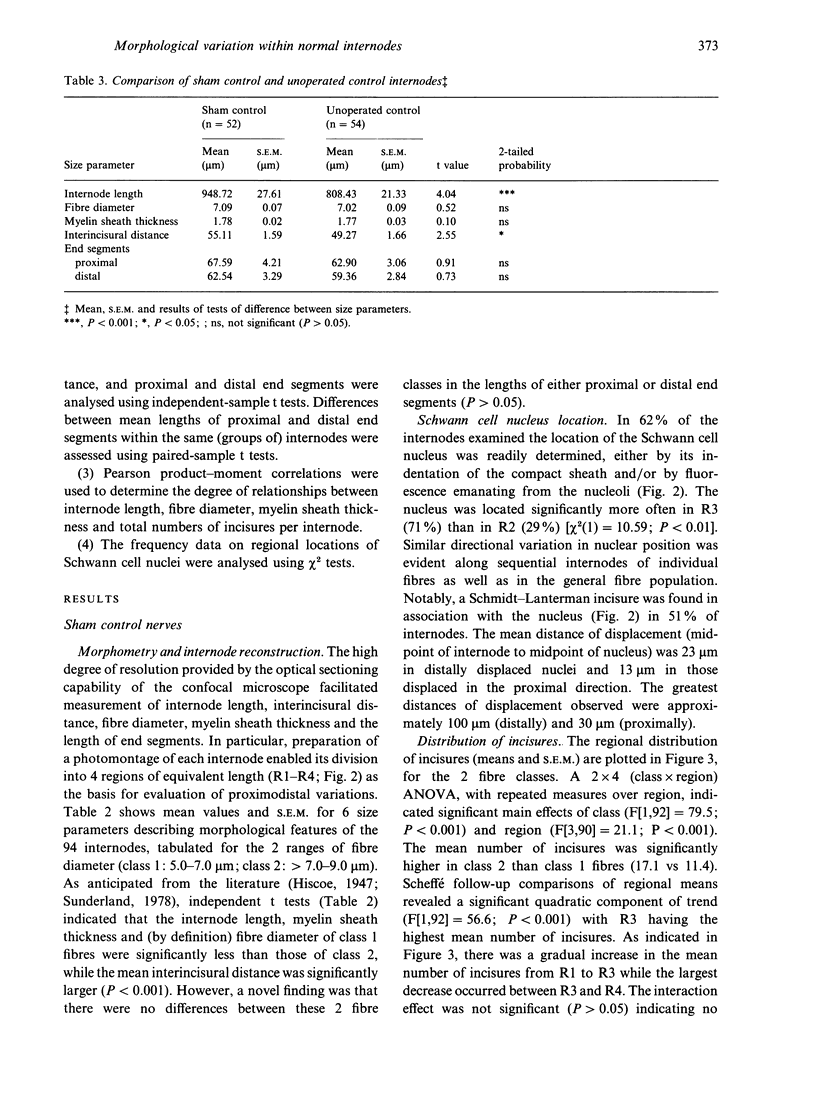
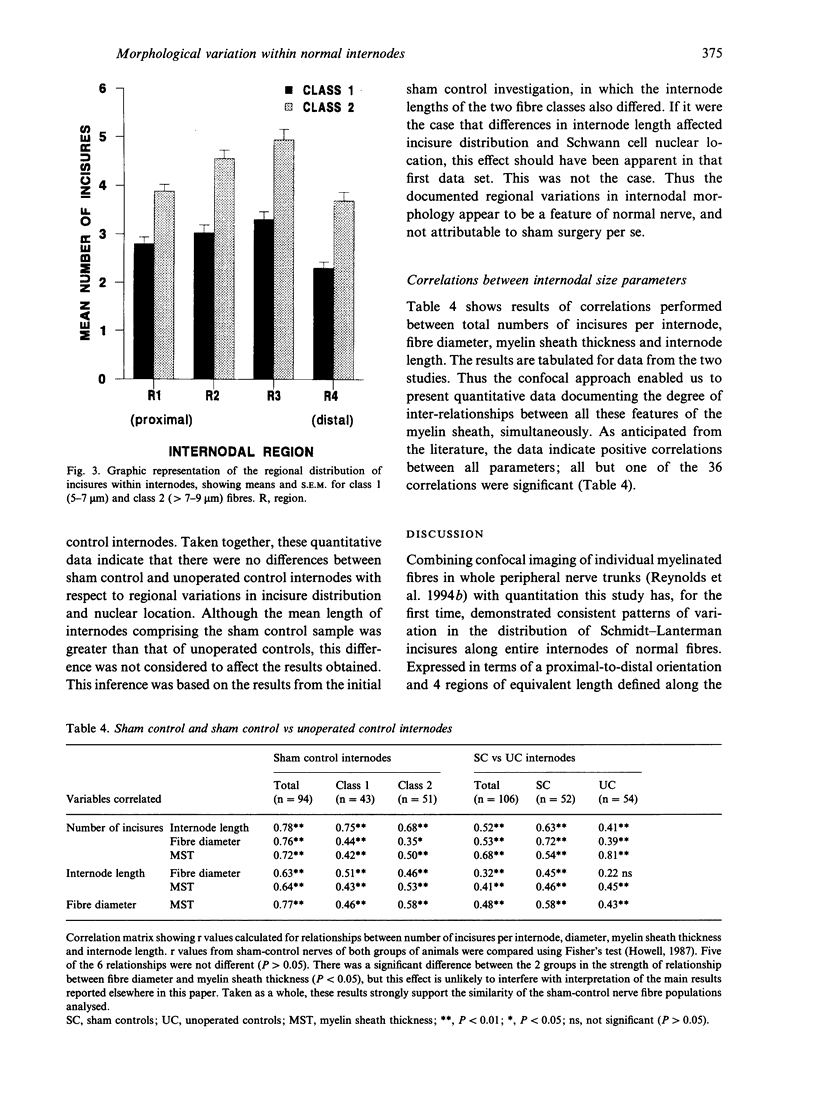
Knowledge of variations in the morphology of normal myelinated peripheral nerve fibres is fundamental to subsequent interpretation of neuropathology. It would be advantageous for structural analysis of normal variations to be based on entire myelin internodes, but acquisition of such data via the classic approach of nerve fibre teasing has been hindered by limitations in optical resolution and specimen preparation. This study addressed these limitations through a new confocal imaging method which permits detailed visualisation of individual myelinated fibres in intact peripheral nerve trunks, and quantitated previously unrecognised patterns of morphological variation within normal internodes. The study focused particularly on Schmidt-Lanterman incisures, the narrow cytoplasmic channels which traverse normal compact myelin and provide foci for disruption of the compact sheath in a number of peripheral neuropathies. Analysis was based on confocal fluorescence images of multiple sequential internodes, traced within posterior tibial nerve trunks of adult male Sprague-Dawley rats. The strength of relationships between internodal size variables (length, fibre diameter, myelin sheath thickness) and total number of incisures per internode were documented. Each internode was divided into 4 regions of equivalent length (regions 1-4), and variations in the distribution of incisures and Schwann cell nuclear location were evaluated. Regional variations were consistent, irrespective of differences in fibre diameter, myelin sheath thickness, and internodal length. Expressed in terms of proximodistal orientation, there was a unimodal distribution of incisures within internodes of this fibre population (diameter range 5-9 microns), with region 3 containing the highest number of incisures and region 4 the lowest (P < 0.05). The Schwann cell nucleus was located more frequently in region 3 than in region 2 (P < 0.01). Contrary to previous reports, an incisure was found in close association with the nucleus in at least 50% of internodes. Documentation of frequent incisure-nuclear association and consistent patterns of variation within internodes extends knowledge of the microanatomy of normal peripheral nerve, and may provide insight into the functional role of incisures. Demonstration of such patterns in normal nerve may contribute to the understanding of pathological change, for example progression of ovoid formation from midinternodal regions during wallerian degeneration.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Azzarelli B., Woodburn R., Olivelle S., Kimbro S., Siakotos A., Taylor M., Lee C. H., Yen M., Paulsrud J. The A-1 antigen: a novel marker in experimental peripheral nerve injury. J Comp Neurol. 1993 Nov 15;337(3):353–365. doi: 10.1002/cne.903370302. [DOI] [PubMed] [Google Scholar]
- Fraher J. P. A quantitative study of anterior root fibres during early myelination. II. Longitudinal variation in sheath thickness and axon circumference. J Anat. 1973 Sep;115(Pt 3):421–444. [PMC free article] [PubMed] [Google Scholar]
- Friede R. L., Samorajski T. Myelin formation in the sciatic nerve of the rat. A quantitative electron microscopic, histochemical and radioautographic study. J Neuropathol Exp Neurol. 1968 Oct;27(4):546–570. [PubMed] [Google Scholar]
- Furness J. B., Heath J. W., Costa M. Aqueous aldehyde (Faglu) methods for the fluorescence histochemical localization of catecholamines and for ultrastructural studies of central nervous tissue. Histochemistry. 1978 Sep 28;57(4):285–295. doi: 10.1007/BF00492664. [DOI] [PubMed] [Google Scholar]
- Gabreëls-Festen A. A., Joosten E. M., Gabreëls F. J., Stegeman D. F., Vos A. J., Busch H. F. Congenital demyelinating motor and sensory neuropathy with focally folded myelin sheaths. Brain. 1990 Dec;113(Pt 6):1629–1643. doi: 10.1093/brain/113.6.1629. [DOI] [PubMed] [Google Scholar]
- Ghabriel M. N., Allt G. Incisures of Schmidt-Lanterman. Prog Neurobiol. 1981;17(1-2):25–58. doi: 10.1016/0301-0082(81)90003-4. [DOI] [PubMed] [Google Scholar]
- Ghabriel M. N., Allt G. Schmidt-Lanterman Incisures. I. A quantitative teased fibre study of remyelinating peripheral nerve fibres. Acta Neuropathol. 1980;52(2):85–95. doi: 10.1007/BF00688005. [DOI] [PubMed] [Google Scholar]
- Ghabriel M. N., Allt G. Schmidt-Lanterman incisures. II. A light and electron microscope study of remyelinating peripheral nerve fibres. Acta Neuropathol. 1980;52(2):97–104. doi: 10.1007/BF00688006. [DOI] [PubMed] [Google Scholar]
- Ghabriel M. N., Allt G. The role of Schmidt-Lanterman incisures in Wallerian degeneration. I. A quantitative teased fibre study. Acta Neuropathol. 1979 Nov;48(2):93–93. [PubMed] [Google Scholar]
- Ghabriel M. N., Allt G. The role of Schmidt-Lanterman incisures in Wallerian degeneration. II. An electron microscopic study. Acta Neuropathol. 1979 Nov;48(2):95–103. doi: 10.1007/BF00691150. [DOI] [PubMed] [Google Scholar]
- Gould R. M., Byrd A. L., Barbarese E. The number of Schmidt-Lanterman incisures is more than doubled in shiverer PNS myelin sheaths. J Neurocytol. 1995 Feb;24(2):85–98. doi: 10.1007/BF01181552. [DOI] [PubMed] [Google Scholar]
- Heath J. W. Double myelination of axons in the sympathetic nervous system. J Neurocytol. 1982 Apr;11(2):249–262. doi: 10.1007/BF01258246. [DOI] [PubMed] [Google Scholar]
- LUBINSKA L. Form of myelinated nerve fibres. Nature. 1954 May 8;173(4410):867–869. doi: 10.1038/173867a0. [DOI] [PubMed] [Google Scholar]
- Lubińska L. Early course of Wallerian degeneration in myelinated fibres of the rat phrenic nerve. Brain Res. 1977 Jul 8;130(1):47–63. doi: 10.1016/0006-8993(77)90841-1. [DOI] [PubMed] [Google Scholar]
- Murray J. M. Neuropathology in depth: the role of confocal microscopy. J Neuropathol Exp Neurol. 1992 Sep;51(5):475–487. [PubMed] [Google Scholar]
- Ohi T., Kyle R. A., Dyck P. J. Axonal attenuation and secondary segmental demyelination in myeloma neuropathies. Ann Neurol. 1985 Mar;17(3):255–261. doi: 10.1002/ana.410170306. [DOI] [PubMed] [Google Scholar]
- Reynolds R. J., Little G. J., Lin M., Heath J. W. Imaging myelinated nerve fibres by confocal fluorescence microscopy: individual fibres in whole nerve trunks traced through multiple consecutive internodes. J Neurocytol. 1994 Sep;23(9):555–564. doi: 10.1007/BF01262056. [DOI] [PubMed] [Google Scholar]
- Saida K., Saida T., Brown M. J., Silberberg D. H., Asbury A. K. Antiserum-mediated demyelination in vivo: a sequential study using intraneural injection of experimental allergic neuritis serum. Lab Invest. 1978 Nov;39(5):449–462. [PubMed] [Google Scholar]
- Saida T., Saida K., Brown M. J., Silberberg D. H. Peripheral nerve demyelination induced by intraneural injection of experimental allergic encephalomyelitis serum. J Neuropathol Exp Neurol. 1979 Sep;38(5):498–518. doi: 10.1097/00005072-197909000-00005. [DOI] [PubMed] [Google Scholar]
- Schröder J. M., Himmelmann F. Fine structural evaluation of altered Schmidt-Lanterman incisures in human sural nerve biopsies. Acta Neuropathol. 1992;83(2):120–133. doi: 10.1007/BF00308471. [DOI] [PubMed] [Google Scholar]
- Seckel B. R. Enhancement of peripheral nerve regeneration. Muscle Nerve. 1990 Sep;13(9):785–800. doi: 10.1002/mus.880130904. [DOI] [PubMed] [Google Scholar]
- Small J. R., Ghabriel M. N., Allt G. The development of Schmidt-Lanterman incisures: an electron microscope study. J Anat. 1987 Feb;150:277–286. [PMC free article] [PubMed] [Google Scholar]
- Sobue G., Doyu M., Watanabe M., Hayashi F., Mitsuma T. Extensive demyelinating changes in the peripheral nerves of Crow-Fukase syndrome: a pathological study of one autopsied case. Acta Neuropathol. 1992;84(2):171–177. doi: 10.1007/BF00311391. [DOI] [PubMed] [Google Scholar]
- WEBSTER H. F. THE RELATIONSHIP BETWEEN SCHMIDT-LANTERMANN INCISURES AND MYELIN SEGMENTATION DURING WALLERIAN DEGENERATION. Ann N Y Acad Sci. 1965 Mar 31;122:29–38. doi: 10.1111/j.1749-6632.1965.tb20189.x. [DOI] [PubMed] [Google Scholar]
- Williams P. L., Hall S. M. Chronic Wallerian degeneration--an in vivo and ultrastructural study. J Anat. 1971 Sep;109(Pt 3):487–503. [PMC free article] [PubMed] [Google Scholar]



