Abstract
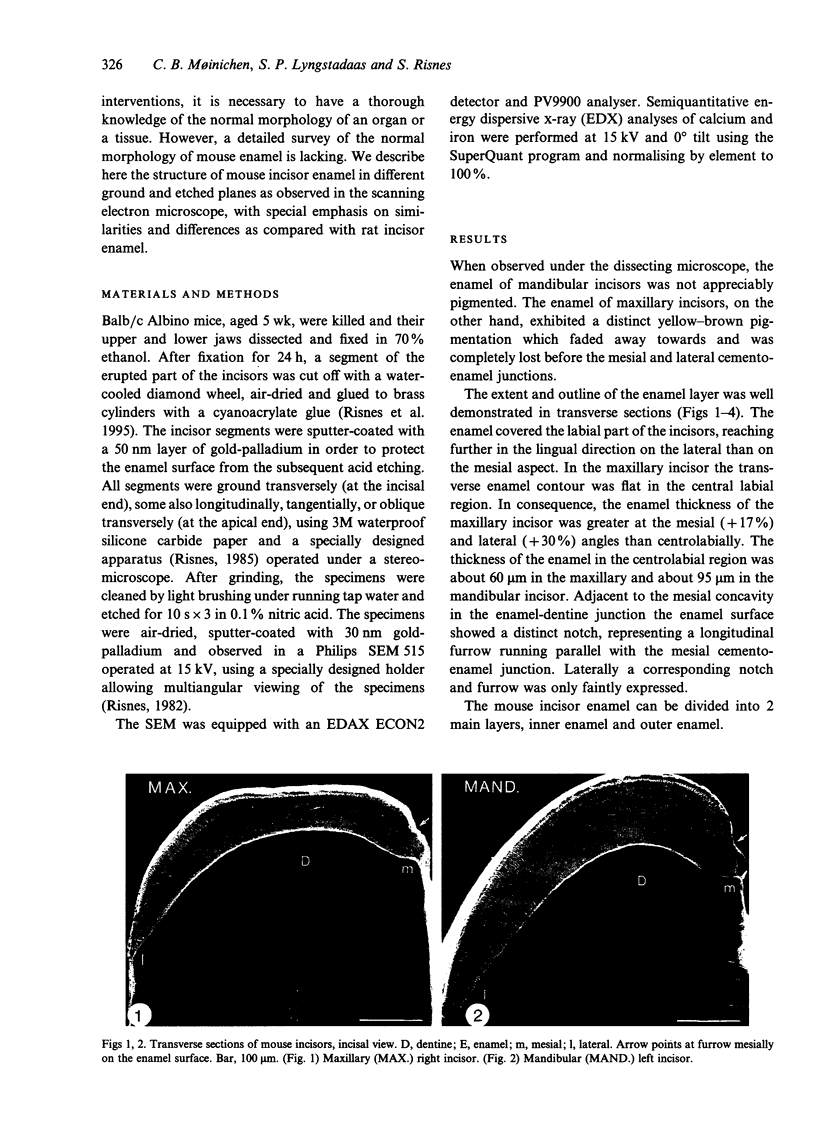
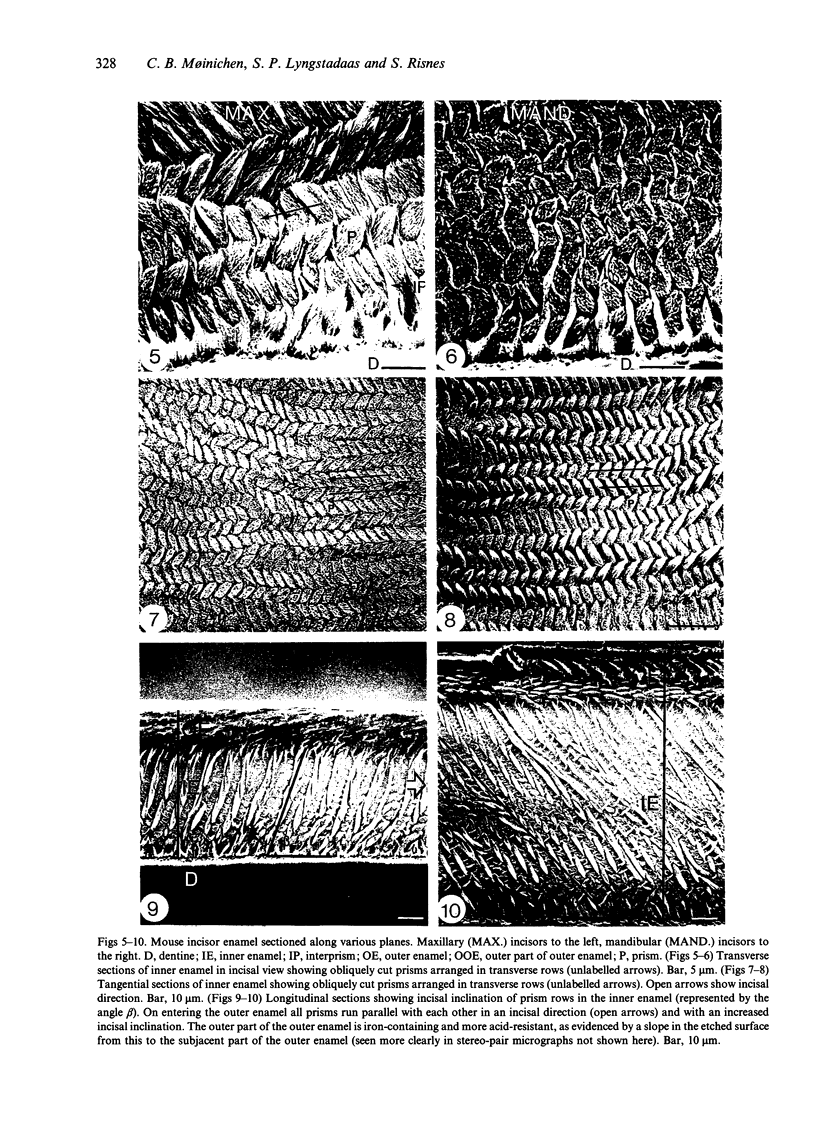
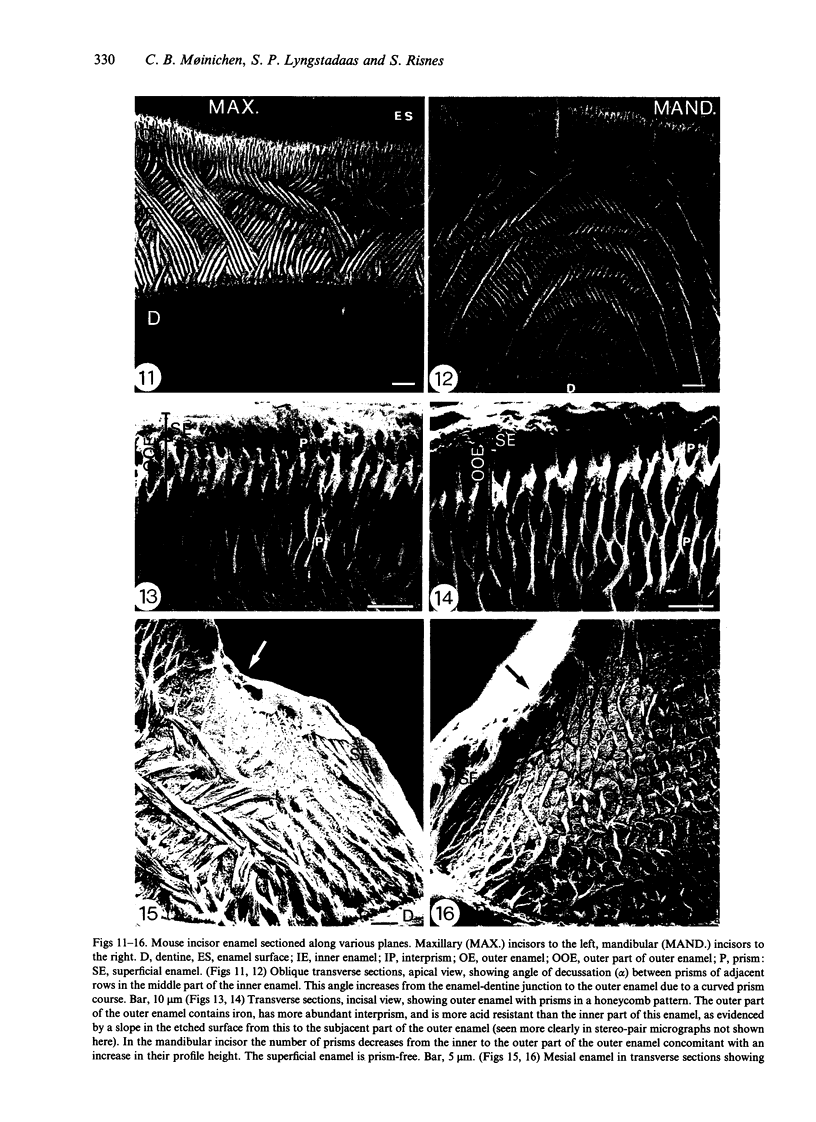
Maxillary and mandibular incisors of mice aged 5 wk were sectioned and ground along various planes, acidetched and observed by scanning electron microscopy (SEM). The general design of the enamel structure resembled rat incisor enamel with an uniserial lamellar pattern of prisms in the inner enamel and incisally directed parallel prisms in the outer enamel. The centrolabial thickness of the enamel was about 60 microns in the maxillary and about 95 microns in the mandibular incisor. The angle between prism rows and enamel-dentine junction was about 70 degrees in the maxillary and about 45 degrees in the mandibular incisor, while the angle of decussation, which increased from the enamel-dentine junction towards the outer enamel, was 50-95 degrees and 30-80 degrees respectively. The angle between outer enamel prisms and enamel surface was about 12 degrees in the maxillary and 5-15 degrees in the mandibular incisor. The outer 1/2-1/3 of the outer enamel contained iron and was more acid-resistant than the rest of the enamel. The superficial 3-5 microns was prismless with a Fe/Ca ratio of about 25/75 in the maxillary and about 10/90 in the mandibular incisor. The latter concentration of iron was insufficient to give visible pigmentation to the enamel. The extreme mesial and lateral enamel was neither typical of inner nor of outer enamel. Assuming that the length of the zone of enamel secretion is half the corresponding length in the rat, it could be calculated that ameloblasts in mouse mandibular incisors produce enamel at a rate of about 6 microns per day, about half the corresponding rate in the rat. In spite of this, the mouse mandibular incisor has a relatively thick layer of enamel, since the ameloblasts spend a relatively long time in the zone of enamel secretion due to a fairly slow eruption rate.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chen E., Piddington R., Decker S., Park J., Yuan Z. A., Abrams W. R., Rosenbloom J., Feldman G., Gibson C. W. Regulation of amelogenin gene expression during tooth development. Dev Dyn. 1994 Mar;199(3):189–198. doi: 10.1002/aja.1001990304. [DOI] [PubMed] [Google Scholar]
- Heap P. F., Berkovitz B. K., Gillett M. S., Thompson D. W. An analytical ultrastructural study of the iron-rich surface layer in rat-incisor enamel. Arch Oral Biol. 1983;28(3):195–200. doi: 10.1016/0003-9969(83)90147-4. [DOI] [PubMed] [Google Scholar]
- Leblond C. P., Warshawsky H. Dynamics of enamel formation in the rat incisor tooth. J Dent Res. 1979 Mar;58(SPEC):950–975. doi: 10.1177/00220345790580024901. [DOI] [PubMed] [Google Scholar]
- Lyngstadaas S. P., Risnes S., Sproat B. S., Thrane P. S., Prydz H. P. A synthetic, chemically modified ribozyme eliminates amelogenin, the major translation product in developing mouse enamel in vivo. EMBO J. 1995 Nov 1;14(21):5224–5229. doi: 10.1002/j.1460-2075.1995.tb00207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matzuk M. M., Kumar T. R., Vassalli A., Bickenbach J. R., Roop D. R., Jaenisch R., Bradley A. Functional analysis of activins during mammalian development. Nature. 1995 Mar 23;374(6520):354–356. doi: 10.1038/374354a0. [DOI] [PubMed] [Google Scholar]
- Ness A. R. Eruption rates of impeded and unimpeded mandibular incisors of the adult laboratory mouse. Arch Oral Biol. 1965 May-Jun;10(3):439–451. doi: 10.1016/0003-9969(65)90109-3. [DOI] [PubMed] [Google Scholar]
- PINDBORG J. J., WEINMANN J. P. Morphologic and functional correlations in the enamel organ of the rat incisor during amelogenesis. Acta Anat (Basel) 1959;36(4):367–381. doi: 10.1159/000141450. [DOI] [PubMed] [Google Scholar]
- Risnes S. A method of calculating the speed of movement of ameloblasts during rat incisor amelogenesis. Arch Oral Biol. 1979;24(4):299–306. doi: 10.1016/0003-9969(79)90092-x. [DOI] [PubMed] [Google Scholar]
- Risnes S. A scanning electron microscope study of aberrations in the prism pattern of rat incisor inner enamel. Am J Anat. 1979 Mar;154(3):419–436. doi: 10.1002/aja.1001540307. [DOI] [PubMed] [Google Scholar]
- Risnes S. Multiangular viewing of dental enamel in the SEM: an apparatus for controlled mechanical specimen preparation. Scand J Dent Res. 1985 Apr;93(2):135–138. doi: 10.1111/j.1600-0722.1985.tb01321.x. [DOI] [PubMed] [Google Scholar]
- Risnes S., Septier D., Goldberg M. Accelerated eruption of rat lower incisor. Relationship between impeded and unimpeded eruption rates, rate of attrition, tooth length, and production of dentin and enamel. Connect Tissue Res. 1995;32(1-4):183–189. doi: 10.3109/03008209509013722. [DOI] [PubMed] [Google Scholar]
- Risnes S. The prism pattern of rat molar enamel: a scanning electron microscope study. Am J Anat. 1979 Jun;155(2):245–257. doi: 10.1002/aja.1001550207. [DOI] [PubMed] [Google Scholar]
- Robinson C., Briggs H. D., Atkinson P. J., Weatherell J. A. Matrix and mineral changes in developing enamel. J Dent Res. 1979 Mar;58(SPEC):871–882. doi: 10.1177/00220345790580024101. [DOI] [PubMed] [Google Scholar]
- Robinson C., Kirkham J., Brookes S. J., Bonass W. A., Shore R. C. The chemistry of enamel development. Int J Dev Biol. 1995 Feb;39(1):145–152. [PubMed] [Google Scholar]
- Salomon J. P., LeGrand J. M., Goldberg M. Scanning electron microscopy of the forming enamel of rat incisor: influence of fixative and treatments interacting with the organic matrix. Scanning Microsc. 1991 Jun;5(2):509–517. [PubMed] [Google Scholar]
- Slavkin H. C., Hu C. C., Sakakura Y., Diekwisch T., Chai Y., Mayo M., Bringas P., Jr, Simmer J., Mak G., Sasano Y. Gene expression, signal transduction and tissue-specific biomineralization during mammalian tooth development. Crit Rev Eukaryot Gene Expr. 1992;2(4):315–329. [PubMed] [Google Scholar]
- Smith C. E., Nanci A. Secretory activity as a function of the development and maturation of ameloblasts. Connect Tissue Res. 1989;22(1-4):147–156. [PubMed] [Google Scholar]
- Smith C. E., Warshawsky H. Movement of entire cell populations during renewal of the rat incisor as shown by radoioautography after labeling with 3H-thymidine. The concept of a continuously differentiating cross-sectional segment. (With an appendix on the development of the periodontal ligament). Am J Anat. 1976 Feb;145(2):225–259. doi: 10.1002/aja.1001450206. [DOI] [PubMed] [Google Scholar]
- Thesleff I., Vaahtokari A., Partanen A. M. Regulation of organogenesis. Common molecular mechanisms regulating the development of teeth and other organs. Int J Dev Biol. 1995 Feb;39(1):35–50. [PubMed] [Google Scholar]
- Warshawsky H. A light and electron microscopic study of the nearly mature enamel of rat incisors. Anat Rec. 1971 Mar;169(3):559–583. doi: 10.1002/ar.1091690307. [DOI] [PubMed] [Google Scholar]
- Warshawsky H., Josephsen K., Thylstrup A., Fejerskov O. The development of enamel structure in rat incisors as compared to the teeth of monkey and man. Anat Rec. 1981 Aug;200(4):371–399. doi: 10.1002/ar.1092000402. [DOI] [PubMed] [Google Scholar]
- Warshawsky H. Radioautographic studies on amelogenesis. J Biol Buccale. 1979 Mar;7(1):105–126. [PubMed] [Google Scholar]
- Warshawsky H., Smith C. E. Morphological classification of rat incisor ameloblasts. Anat Rec. 1974 Aug;179(4):423–446. doi: 10.1002/ar.1091790403. [DOI] [PubMed] [Google Scholar]