Abstract
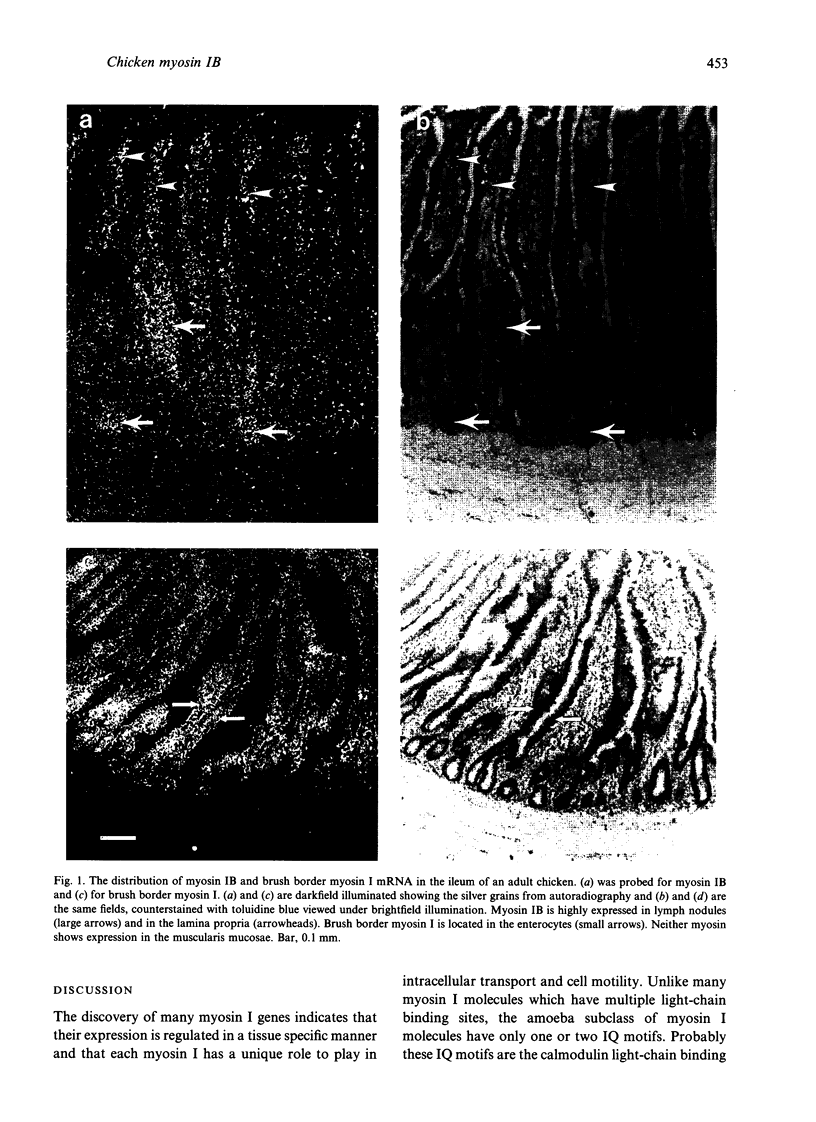
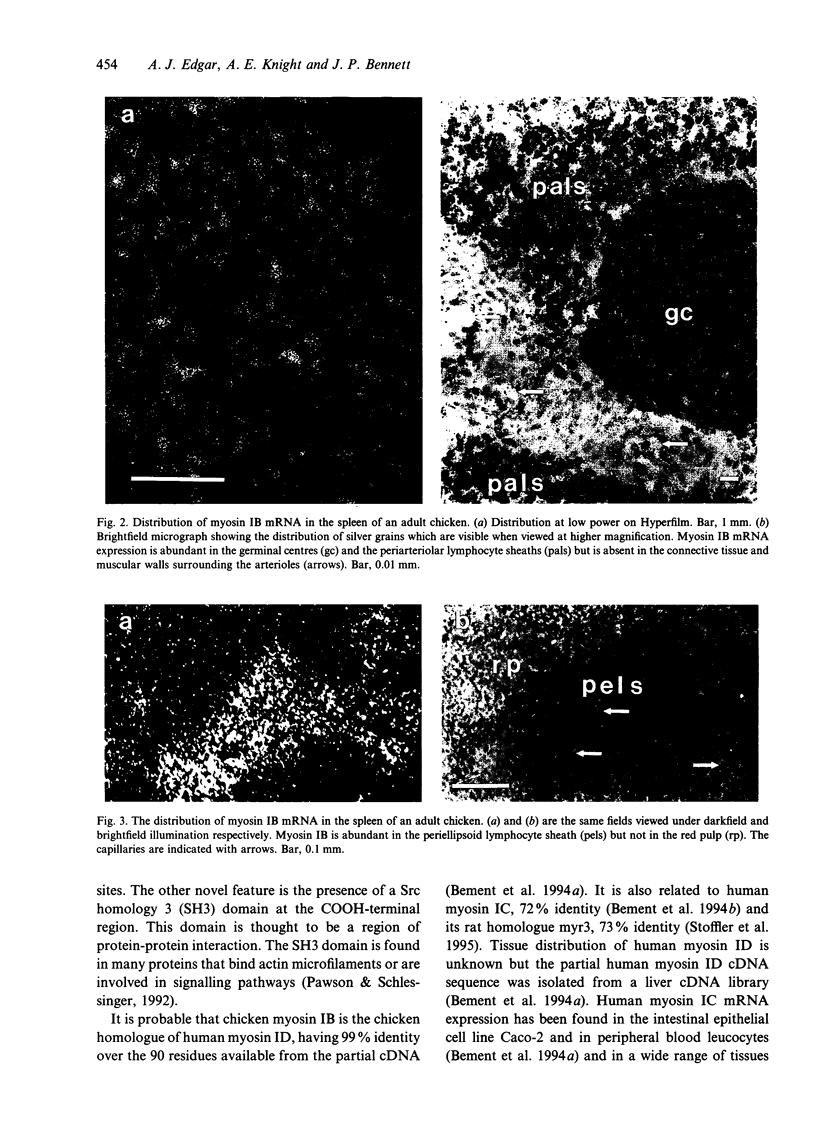
Little is known about the functions of members of the myosin I family in vertebrates. Chicken myosin IB is a member of the amoeba-type subclass of myosin I molecules and tissue localisation studies may provide possible clues to the functions of these myosin I molecules. The expression of the mRNA of this unconventional myosin IB was analysed by in situ hybridization and compared with that of the well characterised brush border myosin I on frozen sections of tissues from the adult domestic chicken. High levels of myosin IB mRNA were found in the intestine and spleen, but were not found in other tissues examined such as brain, heart, lung, liver and kidney. In the intestine, myosin IB mRNA was much more abundant in the lamina propria than in the enterocytes, whereas brush border myosin I mRNA was restricted to the enterocytes. In the spleen, myosin IB mRNA expression was abundant in regions of white pulp, namely germinal centres, periellipsoid lymphocyte sheaths and periarteriolar lymphocyte sheaths. Lymphocytes are the major cell type in both the lamina propria and the white pulp of the spleen, which suggests that chicken myosin IB is highly expressed in lymphocytes. Lymphocyte recirculation depends on their migration through the endothelial layer and it is possible that myosin IB may have a role to play in this type of cell motility.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baines I. C., Brzeska H., Korn E. D. Differential localization of Acanthamoeba myosin I isoforms. J Cell Biol. 1992 Dec;119(5):1193–1203. doi: 10.1083/jcb.119.5.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baines I. C., Corigliano-Murphy A., Korn E. D. Quantification and localization of phosphorylated myosin I isoforms in Acanthamoeba castellanii. J Cell Biol. 1995 Aug;130(3):591–603. doi: 10.1083/jcb.130.3.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bement W. M., Hasson T., Wirth J. A., Cheney R. E., Mooseker M. S. Identification and overlapping expression of multiple unconventional myosin genes in vertebrate cell types. Proc Natl Acad Sci U S A. 1994 Jul 5;91(14):6549–6553. doi: 10.1073/pnas.91.14.6549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bement W. M., Wirth J. A., Mooseker M. S. Cloning and mRNA expression of human unconventional myosin-IC. A homologue of amoeboid myosins-I with a single IQ motif and an SH3 domain. J Mol Biol. 1994 Oct 21;243(2):356–363. doi: 10.1006/jmbi.1994.1662. [DOI] [PubMed] [Google Scholar]
- Bikle D. D., Munson S., Mancianti M. L. Limited tissue distribution of the intestinal brush border myosin I protein. Gastroenterology. 1991 Feb;100(2):395–402. doi: 10.1016/0016-5085(91)90208-3. [DOI] [PubMed] [Google Scholar]
- Bähler M., Kroschewski R., Stöffler H. E., Behrmann T. Rat myr 4 defines a novel subclass of myosin I: identification, distribution, localization, and mapping of calmodulin-binding sites with differential calcium sensitivity. J Cell Biol. 1994 Jul;126(2):375–389. doi: 10.1083/jcb.126.2.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheney R. E., Riley M. A., Mooseker M. S. Phylogenetic analysis of the myosin superfamily. Cell Motil Cytoskeleton. 1993;24(4):215–223. doi: 10.1002/cm.970240402. [DOI] [PubMed] [Google Scholar]
- Coudrier E., Reggio H., Louvard D. Immunolocalization of the 110,000 molecular weight cytoskeletal protein of intestinal microvilli. J Mol Biol. 1981 Oct 15;152(1):49–66. doi: 10.1016/0022-2836(81)90095-4. [DOI] [PubMed] [Google Scholar]
- Espreafico E. M., Cheney R. E., Matteoli M., Nascimento A. A., De Camilli P. V., Larson R. E., Mooseker M. S. Primary structure and cellular localization of chicken brain myosin-V (p190), an unconventional myosin with calmodulin light chains. J Cell Biol. 1992 Dec;119(6):1541–1557. doi: 10.1083/jcb.119.6.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia A., Coudrier E., Carboni J., Anderson J., Vandekerkhove J., Mooseker M., Louvard D., Arpin M. Partial deduced sequence of the 110-kD-calmodulin complex of the avian intestinal microvillus shows that this mechanoenzyme is a member of the myosin I family. J Cell Biol. 1989 Dec;109(6 Pt 1):2895–2903. doi: 10.1083/jcb.109.6.2895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenney J. R., Jr, Osborn M., Weber K. The intracellular localization of the microvillus 110K protein, a component considered to be involved in side-on membrane attachment of F-actin. Exp Cell Res. 1982 Mar;138(1):199–205. doi: 10.1016/0014-4827(82)90106-9. [DOI] [PubMed] [Google Scholar]
- Heintzelman M. B., Hasson T., Mooseker M. S. Multiple unconventional myosin domains of the intestinal brush border cytoskeleton. J Cell Sci. 1994 Dec;107(Pt 12):3535–3543. doi: 10.1242/jcs.107.12.3535. [DOI] [PubMed] [Google Scholar]
- Hoshimaru M., Nakanishi S. Identification of a new type of mammalian myosin heavy chain by molecular cloning. Overlap of its mRNA with preprotachykinin B mRNA. J Biol Chem. 1987 Oct 25;262(30):14625–14632. [PubMed] [Google Scholar]
- Jeurissen S. H. Structure and function of the chicken spleen. Res Immunol. 1991 May;142(4):352–355. doi: 10.1016/0923-2494(91)90090-6. [DOI] [PubMed] [Google Scholar]
- Jung G., Saxe C. L., 3rd, Kimmel A. R., Hammer J. A., 3rd Dictyostelium discoideum contains a gene encoding a myosin I heavy chain. Proc Natl Acad Sci U S A. 1989 Aug;86(16):6186–6190. doi: 10.1073/pnas.86.16.6186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung G., Schmidt C. J., Hammer J. A., 3rd Myosin I heavy-chain genes of Acanthamoeba castellanii: cloning of a second gene and evidence for the existence of a third isoform. Gene. 1989 Oct 30;82(2):269–280. doi: 10.1016/0378-1119(89)90052-8. [DOI] [PubMed] [Google Scholar]
- Kawakami H., Moriyoshi K., Utsumi T., Nakanishi S. Structural organization and expression of the gene for bovine myosin I heavy chain. J Biochem. 1992 Mar;111(3):302–309. doi: 10.1093/oxfordjournals.jbchem.a123754. [DOI] [PubMed] [Google Scholar]
- Koslovsky J. S., Qian C., Jiang X., Mercer J. A. Molecular cloning of a mouse myosin I expressed in brain. FEBS Lett. 1993 Apr 5;320(2):121–124. doi: 10.1016/0014-5793(93)80075-6. [DOI] [PubMed] [Google Scholar]
- Morgan N. S., Skovronsky D. M., Artavanis-Tsakonas S., Mooseker M. S. The molecular cloning and characterization of Drosophila melanogaster myosin-IA and myosin-IB. J Mol Biol. 1994 Jun 10;239(3):347–356. doi: 10.1006/jmbi.1994.1376. [DOI] [PubMed] [Google Scholar]
- Pawson T., Schlessingert J. SH2 and SH3 domains. Curr Biol. 1993 Jul 1;3(7):434–442. doi: 10.1016/0960-9822(93)90350-w. [DOI] [PubMed] [Google Scholar]
- Reizes O., Barylko B., Li C., Südhof T. C., Albanesi J. P. Domain structure of a mammalian myosin I beta. Proc Natl Acad Sci U S A. 1994 Jul 5;91(14):6349–6353. doi: 10.1073/pnas.91.14.6349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruppert C., Godel J., Müller R. T., Kroschewski R., Reinhard J., Bähler M. Localization of the rat myosin I molecules myr 1 and myr 2 and in vivo targeting of their tail domains. J Cell Sci. 1995 Dec;108(Pt 12):3775–3786. doi: 10.1242/jcs.108.12.3775. [DOI] [PubMed] [Google Scholar]
- Ruppert C., Kroschewski R., Bähler M. Identification, characterization and cloning of myr 1, a mammalian myosin-I. J Cell Biol. 1993 Mar;120(6):1393–1403. doi: 10.1083/jcb.120.6.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders G., Lichte B., Meyer H. E., Kilimann M. W. cDNA encoding the chicken ortholog of the mouse dilute gene product. Sequence comparison reveals a myosin I subfamily with conserved C-terminal domains. FEBS Lett. 1992 Oct 26;311(3):295–298. doi: 10.1016/0014-5793(92)81123-4. [DOI] [PubMed] [Google Scholar]
- Sherr E. H., Joyce M. P., Greene L. A. Mammalian myosin I alpha, I beta, and I gamma: new widely expressed genes of the myosin I family. J Cell Biol. 1993 Mar;120(6):1405–1416. doi: 10.1083/jcb.120.6.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snow H., Lowrie M. B., Bennett J. P. A postsynaptic GABA transporter in rat spinal motor neurones. Neurosci Lett. 1992 Aug 31;143(1-2):119–122. doi: 10.1016/0304-3940(92)90246-4. [DOI] [PubMed] [Google Scholar]
- Stöffler H. E., Ruppert C., Reinhard J., Bähler M. A novel mammalian myosin I from rat with an SH3 domain localizes to Con A-inducible, F-actin-rich structures at cell-cell contacts. J Cell Biol. 1995 May;129(3):819–830. doi: 10.1083/jcb.129.3.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilney L. G., Mooseker M. S. Actin filament-membrane attachment: are membrane particles involved? J Cell Biol. 1976 Nov;71(2):402–416. doi: 10.1083/jcb.71.2.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner M. C., Barylko B., Albanesi J. P. Tissue distribution and subcellular localization of mammalian myosin I. J Cell Biol. 1992 Oct;119(1):163–170. doi: 10.1083/jcb.119.1.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu T., Ikebe M. A novel myosin I from bovine adrenal gland. FEBS Lett. 1994 Feb 14;339(1-2):31–36. doi: 10.1016/0014-5793(94)80378-1. [DOI] [PubMed] [Google Scholar]





