Abstract
This study presents the first chemical and enantioselective analyses of essential oils (EOs) derived from the leaves of two endemic species, Gynoxys reinaldii Cuatrec. and Gynoxys pulchella (Kunth) Cass., from Loja, Ecuador. The distillation yields, by weight of dry plant material, were 0.04 ± 0.007% for G. reinaldii and 0.03 ± 0.002% for G. pulchella. For both plants, the chemical analyses were conducted by GC-MS (qualitative) and GC-FID (quantitative), on two stationary phases of different polarity (5% phenyl-methylpolysiloxane and polyethylene glycol). The major components of G. reinaldii EO included germacrene D (22.3–22.1%), α-pinene (14.2–14.1%), and (E)-β-caryophyllene (13.6–14.5%). Similarly, G. pulchella EO was characterized by germacrene D (9.5–12.9%), caryophyllene oxide (7.2–6.7%), and n-tricosane (4.9% in both columns). The enantioselective analyses were carried out with two columns, based on 2,3-diacetyl-6-tert-butyldimethylsilyl-β-cyclodextrin and 2,3-diethyl-6-tert-butyldimethylsilyl-β-cyclodextrin, detecting nine chiral terpenes and terpenoids. In G. reinaldii EO, (1S,5S)-(−)-α-pinene, (1S,5S)-(−)-β-pinene, (1S,5S)-(−)-sabinene, (R)-(−)-α-phellandrene, and (R)-(−)-β-phellandrene were enantiomerically pure, whereas cis-linalool oxide, linalool, terpinene-4-ol, and germacrene D were non-racemic mixtures of enantiomers. In G. pulchella, only (R)-(−)-α-phellandrene was enantiomerically pure. The detection of enantiomerically pure compounds may provide insights into the biosynthetic pathways and potential bioactivities of these EOs.
Keywords: asteraceae, mass spectrometry, enantiomeric composition, β-cyclodextrin, sesquiterpene
1. Introduction
Since ancient times, in all cultures worldwide, plants have been the main source of organic chemicals. Drugs, venoms, perfumes, and fats have been obtained from vegetal materials, usually as complex mixtures. Since the discovery of morphine, many pure natural products have been identified in botanical species, characterized by biological activities or physiological properties [1]. However, after two centuries of research, most of the biodiversity in Europe and North America has been investigated, obliging chemists to focus on tropical flora, especially in “megadiverse” countries like Ecuador [2].
Thanks to the novelty of the Ecuadorian flora, our group has been investigating the very wide but poorly studied chemical diversity of this country for more than 20 years [3,4]. Initially interested in discovering new non-volatile compounds, we recently focused also on the description of unprecedented essential oils (EOs), with an emphasis on their chemical and enantiomeric compositions, biological activity, and olfactory profile [5,6,7,8,9,10].
The main objective of the present study is to enhance the knowledge about phytochemistry and chemotaxonomy of genus Gynoxys Cass., which belongs to the family Asteraceae, in the province of Loja (Ecuador). This genus is native to Argentina, Bolivia, Colombia, Ecuador, Peru, and Venezuela, but the country with the most described specimens is Ecuador [11,12,13]. So far, seven Ecuadorian species have been described as a part of this unfunded project [14,15,16,17,18,19].
On the one hand, according to the literature, G. reinaldii is an endemic shrub, growing between 2000 and 3000 m above the sea level. It has been recorded in the provinces of Azuay and Loja [20]. On the other hand, G. pulchella is an endemic tree, growing at an altitude of 3500–4000 m, and recorded in the provinces of Bolívar, Tungurahua, and Loja. This species is also known as Senecio pulchellus Kunth [12,20]. These taxa have no reported medicinal use, and this study provides the first description of their essential oil composition and enantiomeric profile.
2. Results
2.1. Chemical Analyses of the EOs
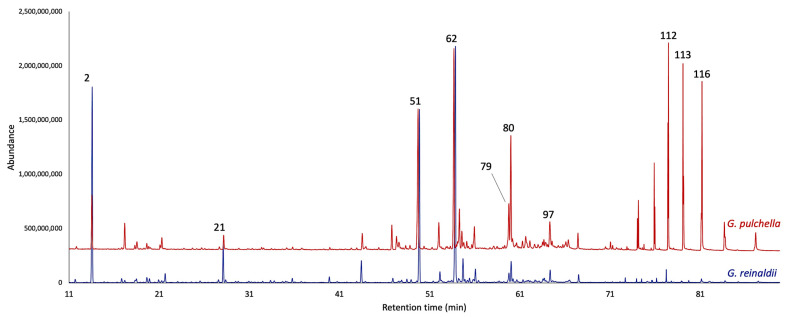
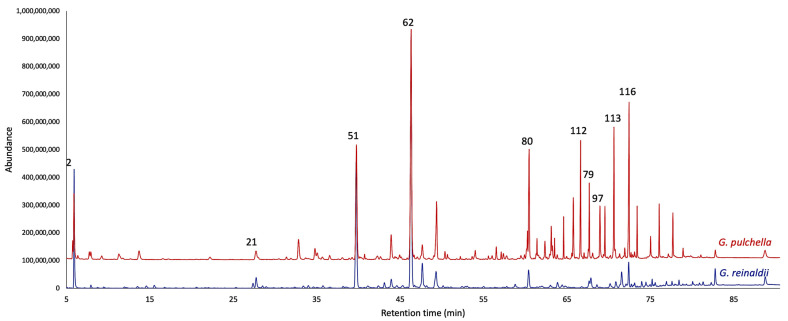
The distillation yields, analytically calculated over four repetitions by weight of dry plant material, were 0.04 ± 0.007% for G. reinaldii and 0.03 ± 0.002% for G. pulchella. The EOs, analyzed on two stationary phases of different polarity, permitted to detect, and quantify a total of 123 compounds, of which only five resulted to be unidentified. In G. reinaldii EO, the total amount of quantified compounds corresponded to 86.3% and 82.8% of the total oil mass on a non-polar and polar column, respectively, whereas in G. pulchella EO, the quantified components altogether corresponded to 90.2% and 91.2%. As is usual for the genus Gynoxys, both essential oils were dominated by the sesquiterpene fraction, including hydrocarbons and oxygenated sesquiterpenoids. About G. reinaldii, the terpene fraction accounted for 54.7–53.4%, whereas G. pulchella EO showed 47.9–50.2%. On the one hand, for G. reinaldii, the second most abundant fraction was constituted by monoterpenes and oxygenated monoterpenoids, whose abundance was 18.7–17.2% on the two columns, respectively. On the other hand, the second main fraction of G. pulchella was composed of non-terpene compounds, principally heavy aliphatic hydrocarbons, whose amount was 34.2–34.3%. The main constituents of G. reinaldii EO (≥3.0 on at least one column) were germacrene D (22.3–22.1%, peak 62), α-pinene (14.2–14.1%, peak 2), (E)-β-caryophyllene (13.6–14.5%, peak 51), n-nonanal (3.0–2.3%, peak 21), and caryophyllene oxide (3.0–3.1%, peak 80). About G. pulchella, the major components were germacrene D (9.5–12.9%, peak 62), caryophyllene oxide (7.2–6.7%, peak 80), (E)-β-caryophyllene (7.0–7.8%, peak 51), n-tricosane (4.9% on both columns, peak 116), 1-docosene (4.0–4.3%, peak 113), α-pinene (3.7–3.0%, peak 2), spathulenol (3.6–3.2%, peak 79), n-heneicosane (3.6–3.5%, peak 112), and α-cadinol (2.1–3.0%, peak 97). The detailed analytical results are shown in Table 1, whereas the GC profiles are reported in Figure 1 and Figure 2.
Table 1.
Qualitative and quantitative compositions of G. reinaldii and G. pulchella EOs.
| N° | Compounds | 5% Phenyl-Methylpolysiloxane | Polyethylene Glycol | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LRI | G. reinaldii | G. pulchella | LRI | G. reinaldii | G. pulchella | Lit. | ||||||||
| Calc. | Ref. [21] | % | σ | % | σ | Calc. | Ref. | % | σ | % | σ | |||
| 1 | heptanal | 911 | 901 | 0.3 | 0.10 | - | - | 1180 | 1180 | 0.4 | 0.08 | - | - | [22] |
| 2 | α-pinene | 934 | 932 | 14.2 | 3.81 | 3.7 | 0.77 | 1015 | 1015 | 14.1 | 3.57 | 3.0 | 0.55 | [23] |
| 3 | α-fenchene | 950 | 945 | 0.1 | 0.02 | - | - | 1053 | 1048 | trace | - | - | - | [24] |
| 4 | thuja-2,4(10)-diene | 955 | 953 | 0.1 | 0.00 | - | - | 1116 | 1116 | 0.3 | 0.08 | - | - | [25] |
| 5 | sabinene | 974 | 969 | 0.3 | 0.08 | 0.1 | 0.02 | 1113 | 1114 | trace | - | 0.1 | 0.03 | [26] |
| 6 | β-pinene | 978 | 974 | 0.2 | 0.05 | 2.0 | 0.46 | 1101 | 1102 | 0.2 | 0.05 | 1.7 | 0.34 | [27] |
| 7 | myrcene | 992 | 988 | 0.1 | 0.03 | 0.2 | 0.05 | 1159 | 1159 | 0.1 | 0.03 | 0.2 | 0.04 | [28] |
| 8 | 2-pentyl furan | 994 | 984 | 0.6 | 0.15 | 0.6 | 0.10 | 1228 | 1229 | 0.5 | 0.08 | 0.2 | 0.10 | [29] |
| 9 | n-decane | 1000 | 1000 | 0.2 | 0.10 | - | - | 1000 | 1000 | trace | - | - | - | - |
| 10 | α-phellandrene | 1008 | 1002 | 0.3 | 0.06 | 0.6 | 0.08 | 1154 | 1153 | 0.3 | 0.09 | 0.2 | 0.04 | [23] |
| 11 | n-octanal | 1012 | 1017 | 0.4 | 0.13 | 0.4 | 0.10 | 1295 | 1295 | 0.2 | 0.04 | trace | - | [30] |
| 12 | (2E,4E)-heptadienal | 1024 | 1005 | 0.5 | 0.08 | 0.1 | 0.02 | 1482 | 1481 | 0.9 | 0.11 | 0.1 | 0.01 | [31] |
| 13 | p-cymene | 1029 | 1022 | 0.3 | 0.16 | 0.7 | 0.14 | 1260 | 1254 | 0.1 | 0.02 | 0.5 | 0.11 | [32] |
| 14 | limonene | 1031 | 1024 | 0.1 | 0.05 | - | - | 1188 | 1189 | 0.1 | 0.01 | - | - | [33] |
| 15 | β-phellandrene | 1033 | 1025 | 0.8 | 0.20 | - | - | 1197 | 1197 | 0.6 | 0.17 | - | - | [34] |
| 16 | (E)-β-ocimene | 1050 | 1044 | 0.1 | 0.03 | - | - | 1248 | 1246 | trace | - | - | - | [33] |
| 17 | cis-linalool oxide (furanoid) | 1075 | 1067 | 0.2 | 0.02 | 0.1 | 0.01 | 1461 | 1465 | trace | - | trace | - | [26] |
| 18 | n-octanol | 1081 | 1063 | 0.2 | 0.03 | - | - | 1555 | 1555 | 0.2 | 0.02 | - | - | [35] |
| 19 | cis-vertocitral C | 1088 | 1076 | - | - | 0.1 | 0.01 | 1206 | - | - | - | trace | - | - |
| 20 | linalool | 1106 | 1095 | 0.3 | 0.02 | 0.2 | 0.02 | 1548 | 1547 | 0.3 | 0.02 | 0.4 | 0.26 | [36] |
| 21 | n-nonanal | 1113 | 1100 | 3.0 | 0.59 | 1.0 | 0.09 | 1386 | 1387 | 2.3 | 0.38 | 0.7 | 0.12 | [37] |
| 22 | cis-β-terpineol | 1131 | 1140 | 0.1 | 0.02 | - | - | 1590 | 1639 | 0.1 | 0.01 | - | - | [38] |
| 23 | α-campholenal | 1135 | 1122 | 0.1 | 0.04 | 0.1 | 0.03 | 1475 | 1472 | 0.2 | 0.05 | trace | - | [39] |
| 24 | citronellal | 1150 | 1148 | 0.2 | 0.06 | - | - | - | - | - | - | - | - | - |
| 25 | verbenol | 1154 | 1140 | 0.2 | 0.04 | - | - | - | - | - | - | - | - | - |
| 26 | isomer of compound 29 | 1159 | 1166 | 0.1 | 0.05 | - | - | 1653 | - | 0.3 | 0.03 | - | - | - |
| 27 | ethyl benzoate | 1167 | 1169 | 0.1 | 0.03 | - | - | - | - | - | - | - | - | - |
| 28 | (2E)-nonen-1-al | 1171 | 1157 | 0.2 | 0.03 | - | - | 1523 | 1524 | 0.2 | 0.02 | - | - | [40] |
| 29 | p-mentha-1,5-dien-8-ol | 1182 | 1185 | 0.3 | 0.05 | - | - | 1718 | 1719 | 0.3 | 0.06 | - | - | [41] |
| 30 | terpinen-4-ol | 1187 | 1174 | 0.2 | 0.03 | trace | - | 1589 | 1589 | 0.1 | 0.01 | trace | - | [42] |
| 31 | n-dodecane | 1200 | 1200 | 0.1 | 0.01 | 0.1 | 0.17 | 1200 | 1200 | trace | - | 0.2 | 0.09 | |
| 32 | γ-terpineol | 1204 | 1199 | 0.2 | 0.09 | - | - | 1709 | - | trace | - | - | - | - |
| 33 | isomer of compound 29 | 1207 | - | 0.1 | 0.02 | - | - | 1771 | - | trace | - | - | - | - |
| 34 | n-decanal | 1215 | 1201 | 0.4 | 0.05 | 0.1 | 0.02 | 1491 | 1493 | 0.4 | 0.03 | 0.5 | 0.14 | [43] |
| 35 | trans-piperitol | 1218 | 1207 | 0.1 | 0.05 | - | - | 1736 | 1738 | 0.1 | 0.04 | - | - | |
| 36 | pulegone | 1228 | 1233 | 0.2 | 0.03 | - | - | - | - | - | - | - | - | - |
| 37 | exo-fenchyl acetate | 1230 | 1229 | - | - | 0.1 | 0.02 | 1457 | 1458 | - | - | 0.6 | 0.15 | [44] |
| 38 | (2E)-decenal | 1272 | 1260 | 0.7 | 0.09 | 0.2 | 0.02 | 1630 | 1630 | 0.6 | 0.08 | 0.4 | 0.04 | [36] |
| 39 | 1-tridecene | 1292 | 1290 | 0.1 | 0.02 | - | - | 1351 | 1352 | 0.1 | 0.01 | - | - | [45] |
| 40 | (2E,4Z)-decadienal | 1306 | 1292 | 0.1 | 0.01 | - | - | 1753 | 1793 | 0.3 | 0.08 | - | - | [46] |
| 41 | p-vinyl guaiacol | 1324 | 1309 | 2.0 | 0.17 | 1.4 | 0.14 | 2186 | 2187 | 2.2 | 0.15 | 0.8 | 0.35 | [47] |
| 42 | (2E,4E)-decadienal | 1331 | 1315 | 0.2 | 0.04 | 0.4 | 0.02 | 1794 | 1795 | 0.4 | 0.03 | 0.7 | 0.03 | [48] |
| 43 | α-cubebene | 1347 | 1348 | 0.1 | 0.02 | - | - | 1521 | 1521 | 0.1 | 0.05 | - | - | [49] |
| 44 | α-ylangene | 1376 | 1373 | 0.4 | 0.01 | 1.4 | 0.14 | 1472 | 1472 | 0.3 | 0.02 | 1.4 | 0.19 | [23] |
| 45 | β-bourbonene | 1384 | 1387 | - | - | 1.2 | 0.28 | 1487 | 1491 | - | - | 0.8 | 0.05 | [26] |
| 46 | (E)-β-damascenone | 1386 | 1383 | 0.2 | 0.04 | 0.2 | 0.04 | 1802 | 1802 | 0.1 | 0.05 | 0.4 | 0.11 | [50] |
| 47 | β-cubebene | 1389 | 1387 | - | - | trace | - | 1469 | 1468 | - | - | 0.2 | 0.04 | [51] |
| 48 | β-Elemene | 1391 | 1389 | 0.5 | 0.04 | - | - | 1597 | 1596 | s.p. 123 | - | - | - | [52] |
| 49 | n-tetradecane | 1400 | 1400 | 0.2 | 0.01 | 0.4 | 0.04 | 1400 | 1400 | 0.3 | 0.03 | 0.1 | 0.02 | |
| 50 | α-gurjunene | 1407 | 1409 | 0.3 | 0.02 | - | - | 1508 | 1507 | trace | - | - | - | [53] |
| 51 | (E)-β-caryophyllene | 1422 | 1417 | 13.6 | 1.42 | 7.0 | 0.77 | 1574 | 1575 | 14.5 | 1.1 | 7.8 | 1.56 | [33] |
| 52 | β-copaene | 1432 | 1430 | 0.1 | 0.00 | 0.2 | 0.02 | 1521 | 1522 | 0.1 | 0.02 | 0.0 | 0.01 | [23] |
| 53 | sesquisabinene | 1456 | 1457 | 0.1 | 0.02 | - | - | 1661 | 1648 | s.p. 65 | - | - | - | [54] |
| 54 | unidentified (MW = 204) | 1458 | 1452 | - | - | 1.8 | 0.22 | 1271 | - | - | - | 1.9 | 0.57 | - |
| 55 | α-humulene | 1458 | 1452 | 1.1 | 0.07 | - | - | 1644 | 1644 | 1.1 | 0.11 | - | - | [42] |
| 56 | allo-aromadendrene | 1461 | 1458 | 0.1 | 0.01 | 0.1 | 0.02 | 1655 | 1655 | 0.2 | 0.02 | 0.1 | 0.03 | [55] |
| 57 | trans-cadina-1(6),4-diene | 1465 | 1475 | - | - | 0.1 | 0.02 | 1505 | - | - | - | trace | - | - |
| 58 | 9-epi-(E)-caryophyllene | 1466 | 1464 | 0.2 | 0.01 | - | - | 1568 | 1572 | 0.1 | 0.01 | - | - | [56] |
| 59 | 4,5-di-epi-aristolochene | 1473 | 1471 | 0.2 | 0.02 | 0.2 | 0.14 | 1657 | 1665 | s.p. 57 | - | 0.1 | 0.03 | [57] |
| 60 | β-chamigrene | 1476 | 1476 | 0.2 | 0.04 | - | - | - | - | - | - | - | - | - |
| 61 | γ-gurjunene | 1478 | 1475 | - | - | 0.4 | 0.09 | - | - | - | - | - | - | - |
| 62 | germacrene D | 1485 | 1480 | 22.3 | 2.86 | 9.5 | 1.02 | 1685 | 1685 | 22.1 | 2.82 | 12.9 | 2.10 | [42] |
| 63 | (E)-β-ionone | 1488 | 1487 | - | - | 1.3 | 0.18 | 1883 | 1889 | - | - | 0.8 | 0.04 | [58] |
| 64 | cis-β-guaiene | 1491 | 1492 | 0.4 | 0.12 | - | - | 1669 | 1667 | 0.3 | 0.03 | - | - | [59] |
| 65 | widdra-2,4(14)-diene | 1491 | 1481 | - | - | 0.4 | 0.12 | 1554 | - | - | - | 0.6 | 0.10 | - |
| 66 | α-zingiberene | 1494 | 1493 | - | - | 1.2 | 0.12 | 1694 | 1696 | - | - | 2.4 | 0.57 | [60] |
| 67 | γ-amorphene | 1496 | 1495 | 0.1 | 0.02 | - | - | - | - | - | - | - | - | - |
| 68 | bicyclogermacrene | 1499 | 1500 | 2.6 | 0.34 | 1.0 | 0.19 | 1709 | 1707 | 2.3 | 0.32 | 1.2 | 0.25 | [61] |
| 69 | α-muurolene | 1503 | 1500 | 1705 | 1700 | [62] | ||||||||
| 70 | (E,E)-α-farnesene | 1508 | 1505 | 0.4 | 0.05 | 0.4 | 0.03 | 1743 | 1743 | 0.3 | 0.06 | 0.2 | 0.03 | [33] |
| 71 | β-bisabolene | 1511 | 1505 | 0.3 | 0.03 | - | - | 1713 | 1710 | s.p. 69 | - | - | - | [49] |
| 72 | germacrene A | 1511 | 1508 | - | - | 0.7 | 0.33 | - | - | - | - | - | - | - |
| 73 | γ-cadinene | 1518 | 1513 | 0.3 | 0.03 | 0.2 | 0.11 | 1738 | 1738 | 0.4 | 0.05 | 0.1 | 0.04 | [42] |
| 74 | n-tridecanal | 1519 | 1509 | 0.3 | 0.04 | - | - | 1806 | 1805 | 0.2 | 0.04 | - | - | [43] |
| 75 | δ-cadinene | 1522 | 1522 | 1.4 | 0.15 | 1.4 | 0.18 | 1738 | 1737 | 1.5 | 0.17 | 1.1 | 0.25 | [26] |
| 76 | trans-cadina-1,4-diene | 1538 | 1533 | 0.1 | 0.02 | - | - | - | - | - | - | - | - | - |
| 77 | (E)-nerolidol | 1567 | 1561 | 0.2 | 0.02 | - | - | 2036 | 2033 | 0.2 | 0.01 | - | - | [63] |
| 78 | germacrene D-4-ol | 1584 | 1574 | 1.4 | 0.17 | - | - | 2042 | 2044 | 0.1 | 0.01 | - | - | [64] |
| 79 | spathulenol | 1585 | 1577 | 3.6 | 0.66 | 2110 | 2106 | 1.3 | 0.26 | 3.2 | 0.19 | [26] | ||
| 80 | caryophyllene oxide | 1589 | 1582 | 3.0 | 0.44 | 7.2 | 1.29 | 1948 | 1944 | 3.1 | 0.32 | 6.7 | 0.75 | [65] |
| 81 | n-hexadecane | 1600 | 1600 | 0.3 | 0.03 | 0.5 | 0.04 | 1600 | 1600 | 0.3 | 0.02 | 0.2 | 0.05 | |
| 82 | ledol | 1612 | 1602 | 0.2 | 0.03 | - | - | 2000 | 2007 | 0.2 | 0.02 | - | - | [66] |
| 83 | unidentified (MW = 222) | 1613 | - | - | - | 0.8 | 0.02 | 2181 | - | - | - | 0.9 | 0.05 | - |
| 84 | β-oplopenone | 1616 | 1607 | 0.2 | 0.01 | - | - | 2039 | 2051 | 0.1 | 0.02 | - | - | [66] |
| 85 | humulene epoxide II | 1619 | 1608 | - | - | 1.3 | 0.04 | 1964 | 1972 | - | - | 0.8 | 0.12 | [67] |
| 86 | n-tetradecanal | 1621 | 1611 | 0.4 | 0.13 | - | - | 1911 | 1910 | trace | - | - | - | [68] |
| 87 | allo-aromadendrene epoxide | 1627 | 1639 | 0.4 | 0.03 | 0.4 | 0.02 | 2093 | 2095 | 0.2 | 0.02 | 0.2 | 0.05 | [69] |
| 88 | unidentified (MW = 220) | 1628 | - | - | - | 0.7 | 0.07 | 1940 | - | - | - | 0.8 | 0.07 | - |
| 89 | junenol | 1630 | 1618 | 0.2 | 0.03 | - | - | - | - | - | - | - | - | - |
| 90 | 1-epi-Cubenol | 1636 | 1627 | 0.3 | 0.04 | 0.4 | 0.02 | 2052 | 2046 | 0.3 | 0.06 | - | - | [70] |
| 91 | cis-cadin-4-en-7-ol | 1644 | 1635 | 0.2 | 0.03 | - | - | 2090 | - | 0.2 | 0.04 | - | - | - |
| 92 | epi-α-cadinol | 1652 | 1638 | 0.5 | 0.24 | 0.7 | 0.06 | 2153 | 2154 | 0.8 | 0.13 | 0.6 | 0.09 | [71] |
| 93 | epi-α-muurolol | 1654 | 1640 | 0.4 | 0.30 | 0.6 | 0.05 | 2170 | 2171 | 0.7 | 0.11 | 0.5 | 0.20 | [59] |
| 94 | α-muurolol (=torreyol) | 1658 | 1645 | - | - | 0.7 | 0.37 | 2150 | 2150 | - | - | 0.5 | 0.20 | [67] |
| 95 | 7-epi-α-eudesmol | 1657 | 1662 | 0.3 | 0.04 | - | - | - | - | - | - | - | - | - |
| 96 | unidentified (MW = 220) | 1660 | - | - | - | 0.7 | 0.05 | 2243 | - | - | - | 0.5 | 0.12 | - |
| 97 | α-cadinol | 1666 | 1652 | 1.6 | 0.22 | 2.1 | 0.13 | 2211 | 2211 | 2.0 | 0.27 | 3.0 | 0.29 | [72] |
| 98 | α-amyl cinnamyl alcohol | 1672 | 1682 | - | - | 0.8 | 0.15 | 2010 | - | - | - | 0.2 | 0.02 | - |
| 99 | ar-turmerone | 1673 | 1668 | 0.1 | 0.02 | - | - | - | - | - | - | - | - | - |
| 100 | n-heptadecane | 1700 | 1700 | 0.3 | 0.03 | 0.6 | 0.07 | - | - | - | - | 0.2 | 0.03 | - |
| 101 | amorpha-4,9-dien-2-ol | 1703 | 1700 | 0.7 | 0.07 | 1.2 | 0.24 | 2343 | - | 0.6 | 0.03 | 1.2 | 0.90 | - |
| 102 | n-pentadecanal | 1725 | 1724 | 0.7 | 0.06 | 1.0 | 0.05 | 2031 | 2024 | 0.7 | 0.19 | 0.4 | 0.09 | [73] |
| 103 | 1-octadecene | 1795 | 1789 | - | - | 0.5 | 0.02 | 1831 | 1823 | - | - | 0.2 | 0.02 | [74] |
| 104 | n-octadecane | 1800 | 1800 | - | - | 0.5 | 0.06 | 1800 | 1800 | - | - | 0.2 | 0.01 | - |
| 105 | cyclopentadecanolide | 1831 | 1832 | 0.1 | 0.02 | - | - | 2255 | 2255 | 0.1 | 0.01 | - | - | [75] |
| 106 | 1-nonadecene | 1895 | 1895 | - | - | 0.7 | 0.03 | 1934 | 1938 | - | - | 0.8 | 0.08 | [76] |
| 107 | n-nonadecane | 1900 | 1900 | 0.1 | 0.05 | 0.8 | 0.05 | 1900 | 1900 | 0.1 | 0.01 | 0.8 | 0.06 | - |
| 108 | (5E,9E)-farnesyl acetone | 1921 | 1913 | 0.1 | 0.06 | - | - | 2368 | 2364 | 0.2 | 0.03 | - | - | [77] |
| 109 | 1-eicosene | 1996 | 1987 | 0.2 | 0.15 | 1.4 | 0.10 | 2057 | 2047 | 0.1 | 0.01 | 1.3 | 0.12 | [78] |
| 110 | n-eicosane | 2000 | 2000 | 0.1 | 0.10 | 0.6 | 0.05 | 2000 | 2000 | trace | - | 2.2 | 0.10 | - |
| 111 | unidentified (MW = 270) | 2095 | - | - | - | 2.4 | 0.24 | 2136 | - | - | - | 2.3 | 0.26 | - |
| 112 | n-heneicosane | 2100 | 2100 | 0.3 | 0.10 | 3.6 | 0.59 | 2100 | 2100 | 0.3 | 0.06 | 3.5 | 0.30 | |
| 113 | 1-docosene | 2196 | 2189 | 0.1 | 0.03 | 4.0 | 0.46 | 2234 | - | 0.2 | 0.08 | 4.3 | 0.95 | - |
| 114 | n-docosane | 2200 | 2200 | - | - | 1.3 | 0.21 | 2200 | 2200 | - | - | 1.6 | 0.18 | |
| 115 | 1-tricosene | 2297 | 2289 | - | - | 1.3 | 0.29 | 2235 | - | - | - | 1.6 | 0.21 | - |
| 116 | n-tricosane | 2300 | 2300 | 0.5 | 0.17 | 4.9 | 0.94 | 2300 | 2300 | 0.4 | 0.01 | 4.9 | 1.46 | |
| 117 | 1-teracosene | 2397 | - | - | - | 1.4 | 0.32 | 2439 | - | - | - | 1.8 | 0.20 | - |
| 118 | n-tetracosane | 2400 | 2400 | 0.1 | 0.01 | 0.5 | 0.12 | 2400 | 2400 | 0.2 | 0.06 | 0.6 | 0.15 | |
| 119 | 1-pentacosene | 2497 | 2486 | - | - | 0.4 | 0.09 | 2547 | - | - | - | 0.6 | 0.13 | - |
| 120 | n-pentacosane | 2500 | 2500 | - | - | 1.2 | 0.41 | 2500 | 2500 | - | - | 0.7 | 0.56 | |
| 121 | 1-hexacosene | 2597 | 2596 * | 0.3 | 0.04 | - | - | 2655 | - | 0.3 | 0.01 | 0.6 | 0.15 | - |
| 122 | n-hexacosane | 2600 | 2600 | 0.2 | 0.02 | trace | - | 2600 | 2600 | 0.2 | 0.02 | 0.6 | 0.20 | |
| 123 | n-tetracosanal | 2637 | 2650 | 0.1 | 0.01 | - | - | 2778 | - | 0.1 | 0.04 | - | - | - |
| monoterpenes | 16.4 | 7.4 | 15.8 | 5.7 | ||||||||||
| oxygenated monoterpenoids | 2.3 | 0.7 | 1.4 | 1.0 | ||||||||||
| sesquiterpenes | 44.8 | 27.5 | 43.4 | 31.3 | ||||||||||
| oxygenated sesquiterpenoids | 9.9 | 20.4 | 10.0 | 18.9 | ||||||||||
| others | 13.0 | 34.2 | 12.2 | 34.3 | ||||||||||
| total | 86.3 | 90.2 | 82.8 | 91.2 | ||||||||||
LRI—Linear Retention Index; Calc.—Calculated; Ref.—Reference; Lit.—Literature; %—Percent amount by weight; σ— Standard deviation; MW—Molecular Weight; * Reference [79].
Figure 1.
Compared GC-MS profiles of G. reinaldii (blue) and G. pulchella (red) EOs from on a 5% phenyl-methylpolysiloxane stationary phase. The numbers refer to peak numbers in Table 1.
Figure 2.
Compared GC-MS profiles of G. reinaldii (blue) and G. pulchella (red) EOs on a polyethylene glycol stationary phase. The numbers refer to peak numbers in Table 1.
2.2. Enantioselective Analyses
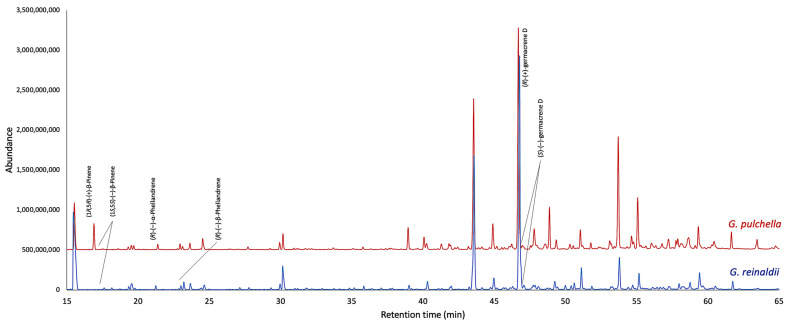
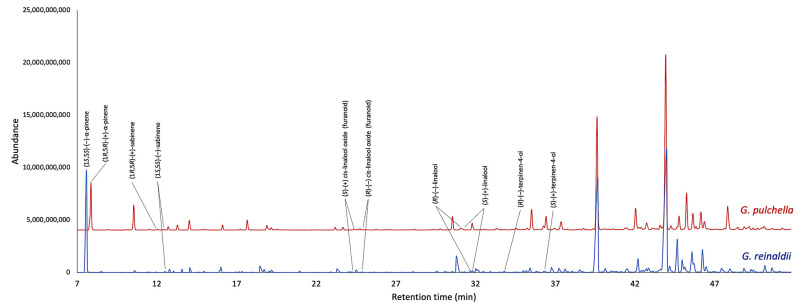
The enantioselective analyses, depending on the EOs chemical composition and the availability of enantiomerically pure standards, were carried out on nine chiral terpenes and terpenoids, whose results are detailed in Table 2, whereas the GC profiles are represented in Figure 3 and Figure 4. In G. reinaldii EO, (1S,5S)-(−)-α-pinene, (1S,5S)-(−)-β-pinene, (1S,5S)-(−)-sabinene, (R)-(−)-α-phellandrene, and (R)-(−)-β-phellandrene were enantiomerically pure, whereas cis-linalool oxide, linalool, terpinene-4-ol, and germacrene D were scalemic mixtures, with linalool almost racemic. About G. pulchella EO, only (R)-(−)-α-phellandrene was enantiomerically pure. In both oils, as is usual in many Gynoxys species, germacrene D showed a very high enantiomeric excess (>96%).
Table 2.
Enantioselective analyses of G. reinaldii and G. pulchella EOs on 2,3-diacetyl-6-tert-butyldimethylsilyl-β-cyclodextrin and 2,3-diethyl-6-tert-butyldimethylsilyl-β-cyclodextrin stationary phases.
| Enantiomers | LRI | G. reinaldii | G. pulchella | ||
|---|---|---|---|---|---|
| Composition | ee (%) | Composition | ee (%) | ||
| (1S,5S)-(−)-α-pinene | 926 a | 100.0 | 100.0 | 12.0 * | 76.0 |
| (1R,5R)-(+)-α-pinene | 928 a | - | 88.0 * | ||
| (1R,5R)-(+)-β-pinene | 949 b | - | 100.0 | 96.9 | 93.8 |
| (1S,5S)-(−)-β-pinene | 959 b | 100.0 | 3.1 | ||
| (1R,5R)-(+)-sabinene | 1008 a | - | 100.0 | 66.7 | 33.4 |
| (1S,5S)-(−)-sabinene | 1014 a | 100.0 | 33.3 | ||
| (R)-(−)-α-phellandrene | 1019 b | 100.0 | 100.0 | 100.0 | 100.0 |
| (S)-(+)-α-phellandrene | 1024 b | - | - | ||
| (R)-(−)-β-phellandrene | 1051 b | 100.0 | 100.0 | - | - |
| (S)-(+)-β-phellandrene | 1058 b | - | - | ||
| (R)-(−)-cis-linalool oxide (furanoid) | 1209 a | 40.7 | 18.6 | 32.4 | 35.2 |
| (S)-(+)-cis-linalool oxide (furanoid) | 1197 a | 59.3 | 67.6 | ||
| (R)-(−)-linalool | 1300 a | 51.5 | 3.0 | 62.7 | 25.4 |
| (S)-(+)-linalool | 1301 a | 48.5 | 37.3 | ||
| (R)-(−)-terpinen-4-ol | 1338 a | 65.1 | 30.2 | - | - |
| (S)-(+)-terpinen-4-ol | 1379 a | 34.9 | - | ||
| (R)-(+)-germacrene D | 1466 b | 98.8 | 97.6 | 98.4 | 96.8 |
| (S)-(−)-germacrene D | 1471 b | 1.2 | 1.6 | ||
a 2,3-diacetyl-6-tert-butyldimethylsilyl-β-cyclodextrin; b 2,3-diethyl-6-tert-butyldimethylsilyl-β-cyclodextrin; * partially resolved.
Figure 3.
Compared GC-MS profiles of G. reinaldii (blue) and G. pulchella (red) EOs on a 2,3-diethyl-6-tert-butyldimethylsilyl-β-cyclodextrin stationary phase.
Figure 4.
Compared GC-MS profiles of G. reinaldii (blue) and G. pulchella (red) EOs on a 2,3-diacethyl-6-tert-butyldimethylsilyl-β-cyclodextrin stationary phase.
3. Discussion
According to the literature, the genus Gynoxys comprises approximately 130 species, of which 34 are recorded in Ecuador and at least 23 are endemic [13,20]. In the present project, 12 species were selected in the province of Loja to be submitted to EO analysis: Gynoxys miniphylla Cuatrec., Gynoxys rugulosa Muschl., Gynoxys buxifolia (Kunth) Cass., Gynoxys laurifolia (Kunth) Cass., Gynoxys cuicochensis Cuatrec., Gynoxys sancti-antonii Cuatrec., Gynoxys szyszylowiczii Hieron., Gynoxys calyculisolvens Hieron., Gynoxys hallii Hieron., Gynoxys azuayensis Cuatrec., Gynoxys pulchella (Kunth) Cass., Gynoxys reinaldii Cuatrec. So far, the EOs of G. miniphylla, G. rugulosa, G. buxifolia, G. laurifolia, G. cuicochensis, G. sancti-antonii, and G. szyszylowiczii have been described and published, whereas G. calyculisolvens, G. hallii, and G. azuayensis are being investigated. Finally, G. pulchella and G. reinaldii are the subjects of the present study [14,15,16,17,18,19].
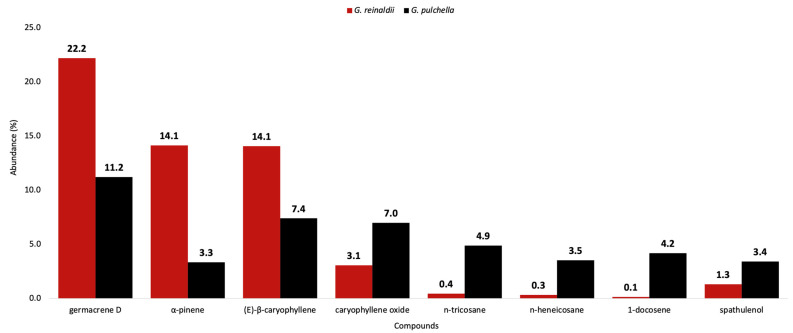
The EOs distilled from the leaves of G. reinaldii and G. pulchella presented quite similar chemical profiles, typical of the volatile fractions of this genus. In fact, as is usual for the Gynoxys spp., three main fractions can be recognized: a poor monoterpene fraction with a strong presence of α-pinene, a dominant sesquiterpene fraction, and a heavy aliphatic fraction [14,15,16,17,18,19]. In Figure 5, the abundances of the main components in these EOs are compared, expressed as mean percent values on the two columns. Germacrene D is the most abundant compound in both oils, whereas (E)-β-caryophyllene is the second main component in G. pulchella and the third one in G. reinaldii. On the other hand, α-pinene is the second major constituent in G. reinaldii. Compositions where these terpenes were present as main components have already been observed in other EOs from this genus, e.g., in the case of G. rugulosa, G. laurifolia, G. szyszylowiczii, and G. cuicochensis [15,17,18,19]. In G. miniphylla, the main EO component is α-phellandrene, whereas in G. sancti-antonii, it is γ-curcumene. However, in all cases, germacrene D, α-pinene, and (E)-β-caryophyllene are present in relatively high amounts [14,15,16,17,18,19]. Regarding the heavy aliphatic fraction, this is very important in G. pulchella, but almost negligible in G. reinaldii. However, other Gynoxys spp. were characterized by an abundant fraction, as it is the case for G. rugulosa and G. szyszylowiczii [15,19]. Anyway, as a trace or in low amount, most of the analyzed Gynoxys EOs presented heavy alkanes and alkenes, confirming that the corresponding biosynthetic pathway is common within this genus.
Figure 5.
Compared abundance of major compounds (≥3.0 in at least one oil) in the EOs of G. reinaldii (red) and G. pulchella (black). Abundances correspond to the mean values of the quantitative results with both columns.
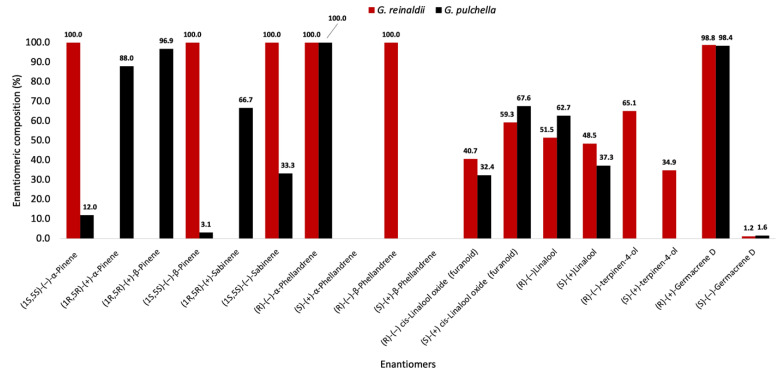
For what concerns the enantiomeric composition of these EOs, a graphical comparison between the two is represented in Figure 6. Unlike the chemical analyses, the enantiomeric profiles were not so similar, with some radical differences among the hydrocarbon monoterpenes. In fact, it can be observed that G. reinaldii produced enantiomerically pure (1S,5S)-(−)-α-pinene and (1S,5S)-(−)-β-pinene, whereas G. pulchella presented high enantiomeric excesses of both dextrorotatory forms. A similar but not identical trend could be observed for sabinene. On the other hand, both species produced the same enantiomer of α-phellandrene, whereas β-phellandrene is absent in G. pulchella and its laevorotatory form is enantiomerically pure in G. reinaldii. These results about pinenes and phellandrenes are somehow biosynthetically consistent. In fact, the stereogenic centres in pinenes are formed when the pinyl cation, the direct precursor of both α-pinene and β-pinene, is produced [80]. Similarly, both α-phellandrene and β-phellandrene derive from the same phellandryl cation, sharing the configuration of the only asymmetric carbon [80]. Although a different trend has sometimes been observed, the expected situation is as described here: the same absolute configuration for α- and β-pinene on one side, and α- and β-phellandrene on the other side, within the same species. About linalool and linalool oxide, the two EOs were instead similar. Not only were these enantiomeric mixtures relatively close to racemic, but, also, in both plants, the enantiomeric excess of linalool oxide was in favor of the dextrorotatory form, whereas for linalool, the laevorotatory isomer was dominant. This phenomenon suggested that linalool oxide could be obtained from linalool through an enantiospecific oxidation. Finally, (R)-(+)-germacrene D presented a very high enantiomeric excess in both plants. This condition, where germacrene D is almost enantiomerically pure, is common in the genus Gynoxys, although sometimes the dominant isomer is dextrorotatory, and at other times, laevorotatory.
Figure 6.
Compared enantiomeric composition of some chiral compounds in the EOs of G. reinaldii (red) and G. pulchella (black).
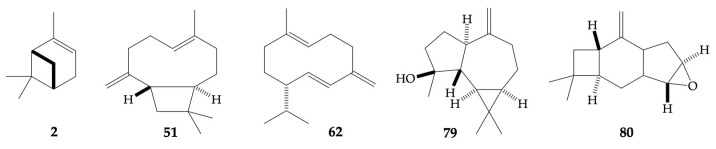
About a possible biological activity of these EOs, the analytical scale distillation did not permit to carry out any assay with this respect. In fact, in the analytical approach, the EO is distilled over an exact volume of cyclohexane, containing an internal standard. The condensed vapors are extracted through the solvent, avoiding the obtention of a pure volatile fraction. The obtained cyclohexane solution can be directly injected into GC, however this approach usually prevents from any application that requires a pure EO, such as conducting a biological activity test. Nevertheless, some literature exists on the properties of the five major terpene constituents (see Figure 7), whose biological activities can be reflected in G. reinaldii and G. pulchella EOs. For what concerns germacrene D (62), the main property reported is an ecologically interesting attractive effect for the moths of the genus Heliothis and Helicoverpa [81]. However, according to further studies, it seems to be the laevorotatory enantiomer the one responsible for this capacity, whereas G. reinaldii and G. pulchella almost exclusively produced (R)-(+)-germacrene D [82,83]. If the role of (R)-(+)-germacrene D versus Heliothis and Helicoverpa spp. can be excluded, the ecological properties of other abundant terpenes and terpenoids must be mentioned. This is for instance the case of α-pinene (2), (E)-β-caryophyllene (51), and caryophyllene oxide (80), whose antifungal, allelopathic, insect-repellent, and insect-attractive effects have been known for more than 40 years [84]. In particular, on the one hand, caryophyllene oxide (80) demonstrated a good fungistatic activity against Pestalotia subcuticularis, a leaf-spotting fungi, pathogen to genus Hymenaea. On the other hand, (E)-β-caryophyllene (51), whose fungistatic activity was very modest, showed important insecticidal capacity against herbivorous lepidoptera, also threatening the genus Hymenaea. If we consider that Hymenaea spp. produce both compounds, whose relative abundance depends on the ecological necessity of the plant, a similar role could be hypothesized for these metabolites in G. reinaldii and G. pulchella EOs.
Figure 7.
Major terpene constituents of G. reinaldii and G. pulchella EOs (≥3.0 in at least one oil, as a mean value on both columns). The numbers refer to Table 1: α-pinene (2), (E)-β-caryophyllene (51), germacrene D (62), spathulenol (79), and caryophyllene oxide (80).
After germacrene D, the second main component of G. reinaldii EO was α-pinene (2), probably one of the pharmacologically most investigated terpenes. This metabolite is known for possessing antibacterial, antifungal, anti-leishmanial, anti-inflammatory, antioxidant, neuroprotective, antitumor, insecticidal, and nematocidal activities, with the anti-inflammatory capacity likely being the most important. As expected, its enantiomers present different biological properties, with (1S,5S)-(−)-α-pinene (G. reinaldii) being anti-viral, whereas (1R,5R)-(+)-α-pinene (G. pulchella) is neuroprotective [85,86]. After that, (E)-β-caryophyllene (51) was the third main constituent in both oils, almost reaching 15% in G. reinaldii (similar to α-pinene) and 7% in G. pulchella (similar to caryophyllene oxide). This very common sesquiterpene hydrocarbon has been widely studied, and its anti-inflammatory, neuroprotective, analgesic, antioxidant, sedative, anxiolytic, and antitumor activities have been described. However, also in this case, the anti-inflammatory and anticancer capacities probably are the most important [87,88]. Caryophyllene oxide (80) was as abundant as (E)-β-caryophyllene in G. pulchella EO. This oxygenated sesquiterpenoid is an epoxide, directly deriving from the oxidation of (E)-β-caryophyllene, with which it shares anticancer activity. This property seems to be more evident in caryophyllene oxide than in (E)-β-caryophyllene, and it has been explained by the electrophilic character of the epoxide group [89]. Furthermore, both (E)-β-caryophyllene and caryophyllene oxide showed an interesting analgesic activity as a consequence of their affinity for the CB2 cannabinoid receptors [89].
Finally, spathulenol (79) was a major oxygenated terpenoid in the EO of G. pulchella. Although this compound has not been extensively studied in a pure form about its pharmacology, there is a significant amount of literature on the biological activities of EOs where spathulenol is the most abundant constituent. This is, for example, the case of Psidium guineense Sw., where spathulenol accounted for more than 80% of the total oil composition. On that occasion, both EO and purified spathulenol demonstrated important anti-inflammatory activity, as well as moderate antiproliferative and antimycobacterial capacities against an ovarian cancer cell line and Mycobacterium tuberculosis, respectively [90].
As is usual within natural products, the presence of both enantiomerically pure or scalemic chiral compounds is the result of enantioselective and enantiospecific biosynthetic pathways. The need for a specific chirality in secondary metabolites depends on the different biological properties associated with different enantiomers. In fact, it is well known that, although two optical isomers are characterized by the same physicochemical properties (except for optical activity), they present different biological capacities due to the chiral medium constituted by living organisms (chiral receptors, chiral active sites, etc.). Therefore, when two enantiomers are produced by the same organism in nature, they usually come from different enantioselective biosynthetic pathways in order to play different biochemical roles.
4. Materials and Methods
4.1. Plant Material
The leaves of G. reinaldii were collected on 28 October 2022, from different shrubs, located in a range of 100 m around a central point of coordinates 03°59′42″ S and 79°16′10″ W, at an altitude of about 2600 m above the sea level. About G. pulchella, the leaves were harvested on June 6, 2023, in a range of about 100 m from a point of coordinates 03°41′36″ S and 79°17′49″ W, at about 3140 m above the sea level. The taxonomic identification was carried out by one of the authors (N.C.), based on the specimens conserved at the Missouri Botanical Garden with codes 3,595,614 (G. reinaldii) and 5,813,849 (G. pulchella). Two botanical vouchers were deposited at the herbarium of the Universidad Técnica Particular de Loja, with codes 14,665 and 14,674 for G. reinaldii and G. pulchella, respectively. Both collection places were located in the province of Loja, Ecuador. After collection, the plant materials were dried at 35 °C for 48 h and stored in dark bags until use. This investigation was conducted in compliance of Ecuadorian law, under the permission of the Ministry of Environment, Water, and Ecological Transition of Ecuador (MAATE), with registry code MAATE-DBI-CM-2022-0248. For both G. reinaldii and G. pulchella, the balsamic period was unknown, and the date of collection was not chosen as a function of the chemical profile or the distillation yield. The determination of the balsamic period would need, for each species, a year-long study that would go beyond the objectives of the present project. For the same reasons, no control has been applied to leaf age so far.
4.2. EOs Distillation and Samples Preparation
Both plants were analytically steam-distilled in four repetitions as previously described in the literature, i.e., using a modified Dean–Stark apparatus. Each repetition was obtained by distilling the plants over 2 mL of cyclohexane containing n-nonane as an internal standard [15]. Both solvent and internal standard were purchased from Merk (Sigma–Aldrich, St. Louis, MO, USA). A total of four samples for each species were obtained, which could be directly injected into the GC. In the distillation of G. reinaldii, each repetition was carried out from 80.0 g of dry plant material, whereas 84.4, 81.6, 51.6, and 52.0 g of dry leaves were used for G. pulchella. All the cyclohexane solutions were stored at −15 °C until use. The distillation apparatus was assembled with commercial glassware, and it was the same equipment used during the whole project for all the Gynoxys spp. Therefore, although it was not the result of a specific design (except for the modified Dean–Stark), it ensured the same reproducible physical parameters and conditions for all the investigated species. Similarly, the distillation yield was not experimentally optimized, but the distillation time was based on the authors’ experience and was maintained the same for all the species in this project.
4.3. Qualitative Analyses (GC-MS) of the EOs
The qualitative analyses were conducted on a Thermo Fisher gas chromatograph (GC) model Trace 1310, coupled with a ISQ 7000 mass spectrometer (MS) from the same provider (Thermo Fisher Scientific, Walthan, MA, USA). The oven was configured with two capillary columns based on the stationary phases of different polarity (30 m long, 0.25 mm internal diameter, and 0.25 μm film thickness). All the analyses were carried out on both phases, 5% phenyl-methylpolysiloxane (TR-5MS, non-polar) and polyethylene glycol (TR-Wax, polar), respectively, provided by Thermo Fisher Scientific (Walthan, MA, USA). The carrier gas was helium, purchased from Indura (Guayaquil, Ecuador), and set at the constant flow of 1 mL/min. All the elutions were conducted according to the following thermal gradient: 50 °C for 10 min, followed by a first gradient of 3 °C/min until 100 °C, a second gradient of 5 °C/min until 200 °C, and a third gradient of 10°C/min until 230 °C, which was maintained for 20 min. The injector was set at 230 °C and operated in split mode (40:1). The MS electron impact (EI) ion source was set at 70 eV and 250 °C, with the mass analyzer operating in SCAN mode (mass range 40–400 m/z), programmed at 250 °C. The transfer line temperature was 230 °C and the injection volume was 1 μL for all samples. Each component of the EOs was identified by comparison of its mass spectrum and linear retention index (LRI) with data from the literature. The LRIs were calculated according to Van den Dool and Kratz, referring to a mixture of homologous n-alkanes in the range C9–C28, purchased from Merk (Sigma–Aldrich, St. Louis, MO, USA) [91]. The use of two stationary phases of different polarity ensured that practically all the detected compounds could be separated with at least on column. Anyway, even when two constituents coeluted on a column, the identification was often successful, thanks to ion mass extraction.
4.4. Quantitative Analyses (GC-FID) of the EOs
The quantitative analyses were conducted with the same GC instrument, columns, thermal program, and conditions as the qualitative ones, but with a flame ionization detector (FID) instead of MS. The split value in all GC-FID analyses was 10:1. With each column, the EOs components were quantified using isopropyl caproate in a six-point calibration curve, where the six dilutions were prepared as previously described in the literature [92]. Before applying the integration areas to the calibration curves, a relative response factor (RRF) was calculated for each compound based on their combustion enthalpy [93,94]. The calibration standard (isopropyl caproate) was synthetized in the authors’ laboratories and purified to 98.8% (GC-FID). The internal standard was n-nonane, purchased from Merk (Sigma–Aldrich, St. Louis, MO, USA). Both calibration curves showed a correlation coefficient >0.998. Because of its dependence from the combustion enthalpy, the RRF value is practically the same for all isomers. Therefore, with FID, an isomer can be used instead of an original compound as a quantification standard. For this reason, we decided to use isopropyl caproate instead of methyl octanoate, which was originally employed by Alain Chaintreau in his method [93].
4.5. Enantioselective Analyses
The enantiomeric compositions of the EOs were determined using two different enantioselective columns, purchased from Mega, Milan, Italy. The column dimensions were 25 m in length, 0.25 mm in internal diameter, and 0.25 μm in phase thickness, whereas the chiral selectors were 2,3-diacetyl-6-tert-butyldimethylsilyl-β-cyclodextrin and 2,3-diethyl-6-tert-butyldimethylsilyl-β-cyclodextrin. The enantioselective analyses were carried out in the same GC-MS instrument and with the same MS settings previously described in Section 4.3, but with the following thermal program: 50 °C for 1 min, followed by a thermal gradient of 2 °C/min until 220 °C, which was maintained for 10 min. The injector and transfer line temperatures were set to 220 °C, whereas the carrier gas flow was set to a constant pressure of 70 kPa. The split value was 40:1, and the injection volume was 1 μL. The enantiomers were identified for their mass spectrum and with the aim of enantiomerically pure standards, injected in the same conditions. A mixture of n-alkanes (C9–C28), provided by Merk (Sigma–Aldrich, St. Louis, MO, USA), was also injected in the same conditions to calculate the retention indices. The chiral selectors were chosen according to the enantiomers that had to be separated and for which enantiomerically pure standards were available.
5. Conclusions
The leaves of Gynoxys reinaldii Cuatrec. and Gynoxys pulchella (Kunth) Cass. produced an EO with a distillation yield of 0.04 ± 0.007% and 0.03 ± 0.002%, respectively. On the one hand, the chemical compositions were relatively similar regarding the terpene fractions, but they were substantially different in the heavy aliphatic fractions. On the other hand, the enantiomeric compositions differed, and, as is usual for the EO of this genus, a common trend was not evident. The only exceptions were linalool and linalool oxide, whose respective enantiomeric compositions were consistent with the hypothesis of an enantiospecific oxidation. According to the chemical compositions, these EOs could be characterized by many biological properties, with the anti-inflammatory activity likely being the most important. Once the present project is complete, all the volatile fractions will be compared using proper statistical analysis.
Acknowledgments
We are grateful to the Universidad Técnica Particular de Loja (UTPL) for supporting this investigation and open access publication. We are also grateful to Carlo Bicchi (University of Turin, Italy) and Stefano Galli (MEGA S.r.l., Legnano, Italy) for their support with enantioselective columns.
Author Contributions
Conceptualization, G.G.; investigation, Y.E.M., M.d.C.R., K.C. and N.C.; data curation, Y.E.M.; writing—original draft preparation, G.G.; writing—review and editing, O.M.; supervision, G.G. All authors have read and agreed to the published version of the manuscript.
Data Availability Statement
The datasets presented in this article are not readily available because they are part of an ongoing study. Requests to access the datasets should be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Huxtable R.J., Schwarz S.K. The isolation of morphine—First principles in science and ethics. Mol. Interv. 2001;1:189–191. [PubMed] [Google Scholar]
- 2.Megadiverse Countries, UNEP-WCMC. [(accessed on 12 June 2024)]. Available online: https://www.biodiversitya-z.org/content/megadiverse-countries.
- 3.Malagón O., Ramírez J., Andrade J., Morocho V., Armijos C., Gilardoni G. Phytochemistry and Ethnopharmacology of the Ecuadorian Flora. A Review. Nat. Prod. Commun. 2016;11:297. doi: 10.1177/1934578X1601100307. [DOI] [PubMed] [Google Scholar]
- 4.Armijos C., Ramírez J., Salinas M., Vidari G., Suárez A.I. Pharmacology and Phytochemistry of Ecuadorian Medicinal Plants: An Update and Perspectives. Pharmaceuticals. 2021;14:1145. doi: 10.3390/ph14111145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chiriboga X., Gilardoni G., Magnaghi I., Vita Finzi P., Zanoni G., Vidari G. New Anthracene Derivatives from Coussarea macrophylla. J. Nat. Prod. 2003;66:905–909. doi: 10.1021/np030066i. [DOI] [PubMed] [Google Scholar]
- 6.Gilardoni G., Tosi S., Mellerio G., Maldonado M.E., Chiriboga X., Vidari G. Lipophilic Components from the Ecuadorian Plant Schistocarpha eupatorioides. Nat. Prod. Commun. 2011;6:767–772. doi: 10.1177/1934578X1100600606. [DOI] [PubMed] [Google Scholar]
- 7.Gilardoni G., Chiriboga X., Finzi P.V., Vidari G. New 3,4-Secocycloartane and 3,4-Secodammarane Triterpenes from the Ecuadorian Plant Coussarea macrophylla. Chem. Biodivers. 2015;12:946–954. doi: 10.1002/cbdv.201400182. [DOI] [PubMed] [Google Scholar]
- 8.Maldonado Y.E., Montalván M., Cumbicus N., Gilardoni G. Chemical and Enantioselective Analyses of an Unprecedented Essential Oil from Ecuadorian Aiouea montana: A Natural Source of S-Methyl-O-2-phenylethyl Carbonothioate. ACS Omega. 2024;9:26495–26502. doi: 10.1021/acsomega.4c02993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gilardoni G., Montalván M., Ortiz M., Vinueza D., Montesinos J.V. The Flower Essential Oil of Dalea mutisii Kunth (Fabaceae) from Ecuador: Chemical, Enantioselective, and Olfactometric Analyses. Plants. 2020;9:1403. doi: 10.3390/plants9101403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Espinosa S., Bec N., Larroque C., Ramírez J., Sgorbini B., Bicchi C., Gilardoni G. Chemical, Enantioselective, and Sensory Analysis of a Cholinesterase Inhibitor Essential Oil from Coreopsis triloba S.F. Blake (Asteraceae) Plants. 2019;8:448. doi: 10.3390/plants8110448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Plants of the World Online Royal Botanic Gardens. [(accessed on 12 June 2024)]. Available online: https://powo.science.kew.org/
- 12.Tropicos.org Missouri Botanical Garden. [(accessed on 12 June 2024)]. Available online: https://www.tropicos.org.
- 13.Arias R., Espinosa-Ortega N., Revilla I., Ansaloni R., Tomasello S. Gynoxys revolutifolia (Senecioneae, Asteraceae): A new species from southern Ecuador. Phytotaxa. 2024;644:211. doi: 10.11646/phytotaxa.644.3.4. [DOI] [Google Scholar]
- 14.Malagón O., Cartuche P., Montaño A., Cumbicus N., Gilardoni G. A New Essential Oil from the Leaves of the Endemic Andean Species Gynoxys miniphylla Cuatrec. (Asteraceae): Chemical and Enantioselective Analyses. Plants. 2022;11:398. doi: 10.3390/plants11030398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maldonado Y.E., Malagón O., Cumbicus N., Gilardoni G. A New Essential Oil from the Leaves of Gynoxys rugulosa Muschl. (Asteraceae) Growing in Southern Ecuador: Chemical and Enantioselective Analyses. Plants. 2023;12:849. doi: 10.3390/plants12040849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cumbicus C., Malagón O., Cumbicus N., Gilardoni G. The Leaf Essential Oil of Gynoxys buxifolia (Kunth) Cass. (Asteraceae): A Good Source of Furanoeremophilane and Bakkenolide A. Plants. 2023;12:1323. doi: 10.3390/plants12061323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gilardoni G., Lara L.R., Cumbicus N., Malagón O. A New Leaf Essential Oil from Endemic Gynoxys laurifolia (Kunth) Cass. of Southern Ecuador: Chemical and Enantioselective Analyses. Plants. 2023;12:2878. doi: 10.3390/plants12152878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maldonado Y.E., Betancourt E.A., León E.S., Malagón O., Cumbicus N., Gilardoni G. New Essential Oils from Ecuadorian Gynoxys cuicochensis Cuatrec. and Gynoxys sancti-antonii Cuatrec. Chemical Compositions and Enantioselective Analyses. ACS Omega. 2024;9:25902–25913. doi: 10.1021/acsomega.4c00391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maldonado Y.E., Malagón O., Cumbicus N., Gilardoni G. A New Leaf Essential Oil from the Andean Species Gynoxys szyszylowiczii Hieron. of Southern Ecuador: Chemical and Enantioselective Analyses. Sci. Rep. 2024;14:16360. doi: 10.1038/s41598-024-67482-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jorgensen P., Leon-Yanez S. Catalogue of the Vascular Plants of Ecuador. Missouri Botanical Garden Press; St. Louis, MO, USA: 1999. pp. 286–288. [Google Scholar]
- 21.Adams R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry. 4th ed. Allured Publishing Corporation; Carol Stream, IL, USA: 2007. [Google Scholar]
- 22.Buttery R.G., Orts W.J., Takeoka G.R., Nam Y. Volatile flavor components of rice cakes. J. Agric. Food Chem. 1999;47:4353–4356. doi: 10.1021/jf990140w. [DOI] [PubMed] [Google Scholar]
- 23.Fanciullino A.L., Gancel A.L., Froelicher Y., Luro F., Ollitrault P., Brillouet J.M. Effects of Nucleo Cytoplasmic Interactions on Leaf Volatile Compounds from Citrus Somatic Diploid Hybrids. J. Agric. Food Chem. 2005;53:4517–4523. doi: 10.1021/jf0502855. [DOI] [PubMed] [Google Scholar]
- 24.Dugo P., Mondello L., Zappia G., Bonaccorsi I., Cotroneo A., Russo M.-T. The composition of the volatile fraction and the enantiomeric distribution of five volatile components of faustrime oil (Monocitrus australatica x Fortunella sp. x Citrus aurantifolia) J. Essent. Oil Res. 2004;16:328–333. doi: 10.1080/10412905.2004.9698734. [DOI] [Google Scholar]
- 25.Flamini G., Tebano M., Cioni P.L., Bagci Y., Dural H., Ertugrul K., Uysal T., Savran A. A Multivariate Statistical Approach to Centaurea Classification Using Essential Oil Composition Data of Some Species from Turkey. Plant Syst. Evol. 2006;261:217–228. doi: 10.1007/s00606-006-0448-3. [DOI] [Google Scholar]
- 26.Yu E.J., Kim T.H., Kim K.H., Lee H.J. Aroma-active compounds of Pinus densiflora (red pine) needles. Flavour Fragr. J. 2004;19:532–537. doi: 10.1002/ffj.1337. [DOI] [Google Scholar]
- 27.Bianchi F., Careri M., Mangia A., Musci M. Retention indices in the analysis of food aroma volatile compounds in temperature programmed gas chromatography: Database creation and evaluation of precision and robustness. J. Sep. Sci. 2007;39:4. doi: 10.1002/jssc.200600393. [DOI] [PubMed] [Google Scholar]
- 28.Ruiz Perez-Cacho P., Mahattanatawee K., Smoot J.M., Rouseff R. Identification of sulfur volatiles in canned orange juices lacking orange flavor. J. Agric. Food Chem. 2007;55:5761–5767. doi: 10.1021/jf0703856. [DOI] [PubMed] [Google Scholar]
- 29.Fernandez-Segovia I., Escriche I., Gomez-Sintes M., Fuentes A., Serra J.-A. Influence of different preservation treatments on the volatile fraction of desalted cod. Food Chem. 2006;98:473–482. doi: 10.1016/j.foodchem.2005.06.021. [DOI] [Google Scholar]
- 30.Mahattanatawee K., Perez-Cacho P.-R., Davenport T., Rouseff R. Comparison of three lychee cultivar odor profiles using gas chromatography-olfactometry and gas chromatography-sulfur detection. J. Agric. Food Chem. 2007;55:1939–1944. doi: 10.1021/jf062925p. [DOI] [PubMed] [Google Scholar]
- 31.Chen C.-C., Kuo M.-C., Liu S.-E., Wu C.-M. Volatile components of salted and pickled prunes (Prunus mume Sieb. et Zucc.) J. Agric. Food Chem. 1986;34:140–144. doi: 10.1021/jf00067a038. [DOI] [Google Scholar]
- 32.Shellie R., Mondello L., Marriott P., Dugo G. Characterization of lavender essential oils by using gas chromatography-mass spectrometry with correlation of linear retention indices and comparison with comprehensive two-dimensional gas chromatography. J. Chromatogr. A. 2002;970:225–234. doi: 10.1016/S0021-9673(02)00653-2. [DOI] [PubMed] [Google Scholar]
- 33.Kim T.H., Thuy N.T., Shin J.H., Baek H.-H., Lee H.J. Aroma-active compounds of miniature beefsteakplant (Mosla dianthera Maxim.) J. Agric. Food Chem. 2000;48:2877–2881. doi: 10.1021/jf000219x. [DOI] [PubMed] [Google Scholar]
- 34.Bader A., Caponi C., Cioni P.L., Flamini G., Morelli I. Acorenone in the essential oil of flowering aerial parts of Seseli tortuosum L. Flavour Fragr. J. 2003;18:57–58. doi: 10.1002/ffj.1154. [DOI] [Google Scholar]
- 35.Wu S., Zorn H., Krings U., Berger R.G. Characteristic Volatiles from Young and Aged Fruiting Bodies of Wild Polyporus sulfureus (Bull.:Fr.) Fr. J. Agric. Food Chem. 2005;53:4524–4528. doi: 10.1021/jf0478511. [DOI] [PubMed] [Google Scholar]
- 36.Cho I.H., Namgung H.-J., Choi H.-K., Kim Y.-S. Volatiles and key odorants in the pileus and stipe of pine-mushroom (Tricholoma matsutake Sing.) Food Chem. 2008;106:71–76. doi: 10.1016/j.foodchem.2007.05.047. [DOI] [Google Scholar]
- 37.Saroglou V., Marin P.D., Rancic A., Veljic M., Skaltsa H. Composition and antimicrobial activity of the essential oil of six Hypericum species from Serbia. Biochem. Syst. Ecol. 2007;35:146–152. doi: 10.1016/j.bse.2006.09.009. [DOI] [Google Scholar]
- 38.Da Porto C., Pizzale L., Bravin M., Conte L.S. Analyses of orange spirit flavour by direct-injection gas chromatography–mass spectrometry and headspace solid-phase microextraction/GC–MC. Flavour Fragr. J. 2003;18:66–72. doi: 10.1002/ffj.1164. [DOI] [Google Scholar]
- 39.Ka M.H., Choi E.H., Chun H.S., Lee K.G. Antioxidative Activity of Volatile Extracts Isolated from Angelica tenuissimae Roots, Peppermint Leaves, Pine Needles, and Sweet Flag Leaves. J. Agric. Food Chem. 2005;53:4124–4129. doi: 10.1021/jf047932x. [DOI] [PubMed] [Google Scholar]
- 40.Riu-Aumatell M., Lopez-Tamames E., Buxaderas S. Assessment of the Volatile Composition of Juices of Apricot, Peach, and Pear According to Two Pectolytic Treatments. J. Agric. Food Chem. 2005;53:7837–7843. doi: 10.1021/jf051397z. [DOI] [PubMed] [Google Scholar]
- 41.Kollmannsberger H., Nitz S., Drawert F. Über die Aromastoffzusammensetzung von Hochdruckextrakten. I. Pfeffer (Piper nigrum, Var. muntok). Z. Für Lebensm. Unters. Und-Forsch. 1992;194:545–551. doi: 10.1007/BF01185481. [DOI] [Google Scholar]
- 42.Yu E.J., Kim T.H., Kim K.H., Lee H.J. Characterization of aroma-active compounds of Abies nephrolepis (Khingan fir) needles using aroma extract dilution analysis. Flavour Fragr. J. 2004;19:74–79. doi: 10.1002/ffj.1314. [DOI] [Google Scholar]
- 43.Tu N.T.M., Onishi Y., Choi H.-S., Kondo Y., Bassore S.M., Ukeda H., Sawamura M. Characteristic odor components of Citrus sphaerocarpa Tanaka (Kabosu) cold-pressed peel oil. J. Agric. Food Chem. 2002;50:2908–2913. doi: 10.1021/jf011578a. [DOI] [PubMed] [Google Scholar]
- 44.Werkhoff P., Güntert M. Identification of some ester compounds in bourbon vanilla beans. Lebensm. Wiss. Technol. 1997;30:429–431. doi: 10.1006/fstl.1996.0194. [DOI] [Google Scholar]
- 45.Tunalier Z., Candan N.T., Demirci B., Baser K.H. The essential oil composition of Acroptilon repens (L.) DC. of Turkish origin. Flavour Fragr. J. 2006;21:462–464. doi: 10.1002/ffj.1670. [DOI] [Google Scholar]
- 46.Pozo-Bayon M.A., Ruiz-Rodriguez A., Pernin K., Cayot N. Influence of eggs on the aroma composition of a sponge cake and on the aroma release in model studies on flavored sponge cakes. J. Agric. Food Chem. 2007;55:1418–1426. doi: 10.1021/jf062203y. [DOI] [PubMed] [Google Scholar]
- 47.Kotseridis Y., Baumes R. Identification of impact odorants in Bordeaux red grape juice, in the commercial yeast used for its fermentation, and in the produced wine. J. Agric. Food Chem. 2000;48:400–406. doi: 10.1021/jf990565i. [DOI] [PubMed] [Google Scholar]
- 48.Mahajan S.S., Goddik L., Qian M.C. Aroma Compounds in Sweet Whey Powder. J. Dairy Sci. 2004;87:4057–4063. doi: 10.3168/jds.S0022-0302(04)73547-X. [DOI] [PubMed] [Google Scholar]
- 49.Flamini G., Cioni P.L., Morelli I., Maccioni S., Baldini R. Phytochemical typologies in some populations of Myrtus communis L. on Caprione Promontory (East Liguria, Italy) Food Chem. 2004;85:599–604. doi: 10.1016/j.foodchem.2003.08.005. [DOI] [Google Scholar]
- 50.Varming C., Andersen M.L., Poll L. Influence of thermal treatment on black currant (Ribes nigrum L. ) juice aroma. J. Agric. Food Chem. 2004;52:7628–7636. doi: 10.1021/jf049435m. [DOI] [PubMed] [Google Scholar]
- 51.Grujic-Jovanovic S., Skaltsa H.D., Marin P., Sokovic M. Composition and antibacterial activity of the essential oil of six Stachys species from Serbia. Flavour Fragr. J. 2004;19:139–144. doi: 10.1002/ffj.1275. [DOI] [Google Scholar]
- 52.Gauvin-Bialecki A., Marodon C. Essential oil of Ayapana triplinervis from Reunion Island: A good natural source of thymohydroquinone dimethyl ether. Biochem. Syst. Ecol. 2009;36:853–858. doi: 10.1016/j.bse.2008.09.006. [DOI] [Google Scholar]
- 53.Shellie R., Marriott P., Zappia G., Mondello L., Dugo G. Interactive use of linear retention indices on polar and apolar columns with an MS-Library for reliable characterization of Australian tea tree and other Melaleuca sp. Oils. J. Essent. Oil Res. 2003;15:305–312. doi: 10.1080/10412905.2003.9698597. [DOI] [Google Scholar]
- 54.Ngassoum M.B., Yonkeu S., Jirovetz L., Buchbauer G., Schmaus G., Hammerschmidt F.-J.H. Chemical composition of essential oils of Lantana camara leaves and flowers from Cameroon and Madagascar. Flavour Fragr. J. 1999;14:245–250. doi: 10.1002/(SICI)1099-1026(199907/08)14:4<245::AID-FFJ819>3.0.CO;2-X. [DOI] [Google Scholar]
- 55.Baser K.H.C., Ozek G., Ozek T., Duran A. Composition of the essential oil of Centaurea huber-morathii Wagenitz isolated from seeds by microdistillation. Flavour Fragr. J. 2006;21:568–570. doi: 10.1002/ffj.1620. [DOI] [Google Scholar]
- 56.Paniandy J.-C., Chane-Ming J., Pierbattesti J.-C. Chemical Composition of the Essential Oil and Headspace Solid-Phase Microextraction of the Guava Fruit (Psidium guajava L.) J. Essent. Oil Res. 2000;12:153–158. doi: 10.1080/10412905.2000.9699486. [DOI] [Google Scholar]
- 57.Cavalli J.-F., Tomi F., Bernardini A.-F., Casanova J. Composition and chemical variability of the bark oil of Cedrelopsis grevei H. Baillon from Madagascar. Flavour Fragr. J. 2003;18:532–538. doi: 10.1002/ffj.1263. [DOI] [Google Scholar]
- 58.Blazevic I., Mastelic J. Free and bounded volatiles of rocket (Eruca sativa Mill.) Fravour Fragr. J. 2008;23:278–285. doi: 10.1002/ffj.1883. [DOI] [Google Scholar]
- 59.Ferretti G., Maggi F., Tirillini B. Essential oil composition of Hypericum richeri Vill. from Italy. Flavour Fragr. J. 2005;20:295–298. doi: 10.1002/ffj.1412. [DOI] [Google Scholar]
- 60.Chen C.-C., Ho C.-T. Gas chromatographic analysis of volatile components of ginger oil (Zingiber officinale Roscoe) extracted with liquid carbon dioxide. J. Agric. Food Chem. 1988;36:322–328. doi: 10.1021/jf00080a020. [DOI] [Google Scholar]
- 61.Brat P., Rega B., Alter P., Reynes M., Brillouet J.-M. Distribution of volatile compounds in the pulp, cloud, and serum of freshly squeezed orange juice. J. Agric. Food Chem. 2003;51:3442–3447. doi: 10.1021/jf026226y. [DOI] [PubMed] [Google Scholar]
- 62.Zheng C.H., Kim K.H., Kim T.H., Lee H.J. Analysis and characterization of aroma-active compounds of Schizandra chinensis (omija) leaves. J. Sci. Food Agric. 2005;85:161–166. doi: 10.1002/jsfa.1975. [DOI] [Google Scholar]
- 63.Wu S., Zorn H., Krings U., Berger R.G. Volatiles from submerged and surface-cultured beefsteak fungus, Fistulina hepatica. Flavour Fragr. J. 2007;22:53–60. doi: 10.1002/ffj.1758. [DOI] [Google Scholar]
- 64.Karlsson M.F., Birgersson G., Prado A.M.C., Bosa F., Bengtsson M., Witzgall P. Plant Odor Analysis of Potato: Response of Guatemalan Moth to Above- and Background Potato Volatiles. J. Agric. Food Chem. 2009;57:5903–5909. doi: 10.1021/jf803730h. [DOI] [PubMed] [Google Scholar]
- 65.Jerkovic I., Mastelic J., Milos M., Juteau F., Masotti V., Viano J. Chemical variability of Artemisia vulgaris L. essential oils originated from the Mediterranean area of France and Croatia. Flavour Fragr. J. 2003;18:436–440. doi: 10.1002/ffj.1246. [DOI] [Google Scholar]
- 66.Hachicha S.F., Skanji T., Barrek S., Ghrabi Z.G., Zarrouk H. Composition of the essential oil of Teucrium ramosissimum Desf. (Lamiaceae) from Tunisia. Flavour Fragr. J. 2007;22:101–104. doi: 10.1002/ffj.1764. [DOI] [Google Scholar]
- 67.Jirovetz L., Buchbauer G., Shafi P.M., Rosamma M.K., Geissler M. Analysis of the composition and aroma of the essential leaf oil of Syzygium travancoricum from South India by GC-FID, GC-MS, and olfactometry. Seasonal changes of composition. Chromatographia. 2001;53:S372–S374. doi: 10.1007/BF02490359. [DOI] [Google Scholar]
- 68.Marques F.A., McElfresh J.S., Millar J.G. Kováts retention indexes of monounsaturated C12, C14, and C16 alcohols, acetates and aldehydes commonly found in lepidopteran pheromone blends. J. Braz. Chem. Soc. 2000;11:592–599. doi: 10.1590/S0103-50532000000600007. [DOI] [Google Scholar]
- 69.Paolini J., Tomi P., Bernardini A.-F., Bradesi P., Casanova J., Kaloustian J. Detailed analysis of the essential oil from Cistus albidus L. by combination of GC/RI, GC/MS and 13C-NMR spectroscopy. Nat. Prod. Res. 2008;22:1270–1278. doi: 10.1080/14786410701766083. [DOI] [PubMed] [Google Scholar]
- 70.Bendiabdellah A., El Amine Dib M., Djabou N., Allali H., Tabti B., Costa J., Myseli A. Biological activities and volatile constituents of Daucus muricatus L. from Algeria. Chem. Cent. J. 2012;6:48. doi: 10.1186/1752-153X-6-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Neves A., Rosa S., Goncalves J., Rufino A., Judas F., Salgueiro L., Lopes M.C., Cavaleiro C., Mendes A.F. Screening of five essential oils for identification of potential inhibitors of IL-1-unduced Nf-kB activation and NO production in human chondrocytes: Characterization of the inhibitory activity of alpha-pinene. Planta Medica. 2010;76:303–308. doi: 10.1055/s-0029-1186085. [DOI] [PubMed] [Google Scholar]
- 72.Choi H.-S. Character impact odorants of Citrus Hallabong [(C. unshiu Marcov × C. sinensis Osbeck) × C. reticulata Blanco] cold-pressed peel oil. J. Agric. Food Chem. 2003;51:2687–2692. doi: 10.1021/jf021069o. [DOI] [PubMed] [Google Scholar]
- 73.Ledauphin J., Basset B., Cohen S., Payot T., Barillier D. Identification of trace volatile compounds in freshly distilled Calvados and Cognac: Carbonyl and sulphur compounds. J. Food Compos. Anal. 2006;19:28–40. doi: 10.1016/j.jfca.2005.03.001. [DOI] [Google Scholar]
- 74.Pennarun A.L., Prost C., Demaimay M. Identification and origin of the character-impact compounds of raw oyster Crassostrea gigas. J. Sci. Food Agric. 2002;82:1652–1660. doi: 10.1002/jsfa.1236. [DOI] [Google Scholar]
- 75.Vinogradov B.A. Production, Composition, Properties and Application of Essential Oils. 2004. [(accessed on 20 November 2024)]. Available online: http://viness.narod.ru.
- 76.Shiratsuchi H., Shimoda M., Imayoshi K., Noda K., Osajima Y. Volatile flavor compounds in spray-dried skim milk powder. J. Agric. Food Chem. 1994;42:984–988. doi: 10.1021/jf00040a028. [DOI] [Google Scholar]
- 77.Kirimer N., Tabanca N., Özek T., Tümen G., Baser K.H.C. Essential oils of annual Sideritis species growing in Turkey. Pharm. Biol. 2000;38:106–111. doi: 10.1076/1388-0209(200004)3821-1FT106. [DOI] [PubMed] [Google Scholar]
- 78.Condurso C., Verzera A., Romeo V., Ziino M., Trozzi A., Ragusa S. The leaf volatile constituents of Isatis tinctoria by solid-phase microextraction and gas chromatography/mass spectrometry. Planta Medica. 2006;72:924–928. doi: 10.1055/s-2006-946679. [DOI] [PubMed] [Google Scholar]
- 79.Radulovic N., Blagojevic P., Palic R. Comparative study of the leaf volatiles of Arctostaphylos uva-ursi (L. ) Spreng. and Vaccinium vitis-idaea L. (Ericaceae). Molecules. 2010;15:6168–6185. doi: 10.3390/molecules15096168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Dewick P.M. Medicinal Natural Products. A Biosynthetic Approach. 3rd ed. John Wiley & Sons Ltd.; Chichester, UK: 2009. [Google Scholar]
- 81.Røstelien T., Borg-Karlson A.K., Fäldt J., Jacobsson U., Mustaparta H. The Plant Sesquiterpene Germacrene D Specifically Activates a Major Type of Antennal Receptor Neuron of the Tobacco Budworm Moth Heliothis virescens. Chem. Senses. 2000;25:141. doi: 10.1093/chemse/25.2.141. [DOI] [PubMed] [Google Scholar]
- 82.Mozuraitis R., Stranden M., Ramirez M.I., Borg-Karlson A.K., Mustaparta H. (-)-Germacrene D Increases Attraction and Oviposition by the Tobacco Budworm Moth Heliothis virescens. Chem. Senses. 2002;27:505–509. doi: 10.1093/chemse/27.6.505. [DOI] [PubMed] [Google Scholar]
- 83.Stranden M., Liblikas I., Koenig W.A., Almaas T.J., Borg-Karlson A.K., Mustaparta H. (-)-GermacreneD Receptor Neurones in Three Species of Heliothine Moths: Structure-activity Relationships. J. Comp. Physiol. A. 2003;189:563–577. doi: 10.1007/s00359-003-0434-y. [DOI] [PubMed] [Google Scholar]
- 84.Langenheim J.H. Higher Plant Terpenoids: A Phytocentric Overview of Their Ecological Roles. J. Chem. Ecol. 1994;20:1223–1280. doi: 10.1007/BF02059809. [DOI] [PubMed] [Google Scholar]
- 85.Allenspach M., Steuer C. α-Pinene: A Never-Ending Story. Phytochemistry. 2021;190:112857. doi: 10.1016/j.phytochem.2021.112857. [DOI] [PubMed] [Google Scholar]
- 86.Salehi B., Upadhyay S., Erdogan Orhan I., Kumar Jugran A., Jayaweera S.L.D., Dias D.A., Sharopov F., Taheri Y., Martins N., Baghalpour N., et al. Therapeutic Potential of α- and β-Pinene: A Miracle Gift of Nature. Biomolecules. 2019;9:738. doi: 10.3390/biom9110738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Francomano F., Caruso A., Barbarossa A., Fazio A., La Torre C., Ceramella J., Mallamaci R., Saturnino C., Iacopetta D., Sinicropi M.S. β-Caryophyllene: A Sesquiterpene with Countless Biological Properties. Appl. Sci. 2019;9:5420. doi: 10.3390/app9245420. [DOI] [Google Scholar]
- 88.Scandiffio R., Geddo F., Cottone E., Querio G., Antoniotti S., Gallo M.P., Maffei M.E., Bovolin P. Protective Effects of (E)-β-Caryophyllene (BCP) in Chronic Inflammation. Nutrients. 2020;12:3273. doi: 10.3390/nu12113273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Fidyt K., Fiedorowicz A., Strządała L., Szumny A. β-Caryophyllene and β-Caryophyllene Oxide—Natural Compounds of Anticancer and Analgesic Properties. Cancer Med. 2016;5:3007–3017. doi: 10.1002/cam4.816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.do Nascimento K.F., Moreira F.M.F., Santos J.A., Kassuya C.A.L., Croda J.H.R., Cardoso C.A.L., Vieira M.D.C., Ruiz A.L.T.G., Foglio M.A., de Carvalho J.E., et al. Antioxidant, Anti-inflammatory, Antiproliferative and Antimycobacterial Activities of the Essential Oil of Psidium guineense Sw. and Spathulenol. J. Ethnopharmacol. 2018;210:351–358. doi: 10.1016/j.jep.2017.08.030. [DOI] [PubMed] [Google Scholar]
- 91.Van Den Dool H., Kratz P.D. A Generalization of the Retention Index System Including Linear Temperature Programmed Gas—Liquid Partition Chromatography. J. Chromatogr. A. 1963;11:463–471. doi: 10.1016/S0021-9673(01)80947-X. [DOI] [PubMed] [Google Scholar]
- 92.Gilardoni G., Matute Y., Ramírez J. Chemical and Enantioselective Analysis of the Leaf Essential Oil from Piper coruscans Kunth (Piperaceae), a Costal and Amazonian Native Species of Ecuador. Plants. 2020;9:791. doi: 10.3390/plants9060791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.De Saint Laumer J.Y., Cicchetti E., Merle P., Egger J., Chaintreau A. Quantification in Gas Chromatography: Prediction of Flame Ionization Detector Response Factors from Combustion Enthalpies and Molecular Structures. Anal. Chem. 2010;82:6457–6462. doi: 10.1021/ac1006574. [DOI] [PubMed] [Google Scholar]
- 94.Tissot E., Rochat S., Debonneville C., Chaintreau A. Rapid GC-FID quantification technique without authentic samples using predicted response factors. Flavour Fragr. J. 2012;27:290–296. doi: 10.1002/ffj.3098. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets presented in this article are not readily available because they are part of an ongoing study. Requests to access the datasets should be directed to the corresponding author.