Abstract
The behaviour of the cow blastocyst in vitro was studied by time-lapse cinematography and analysed by morphometry. Three types of behaviour were observed: continuous expansion followed by hatching; discontinuous expansion interrupted by few contractions and followed by hatching; discontinuous expansion interrupted by several rapid contractions without hatching. This demonstrated that the pulsatile activity of the blastocyst is not a necessary condition of hatching but also that only a moderate pulsatile activity is compatible with normal hatching. The time of hatching in vitro corresponded approximately with the time of zona loss in vivo (9-10 days). Rupture of the zona occurred at any point of the trophoblast layer. Hatching by herniation through a reduced opening of the zona was occasionally observed. The behavior of the embryos from a particular animal was very similar but differences were noted between embryos from different animals.
Full text
PDF

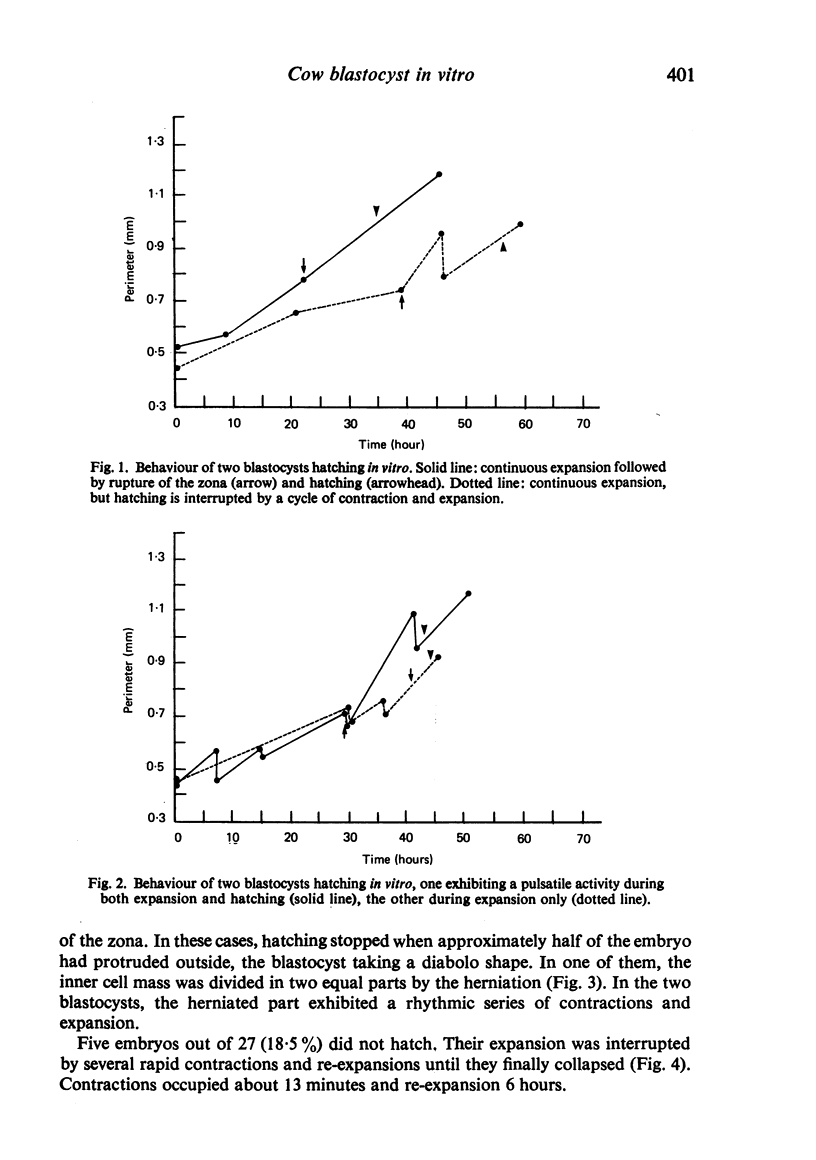
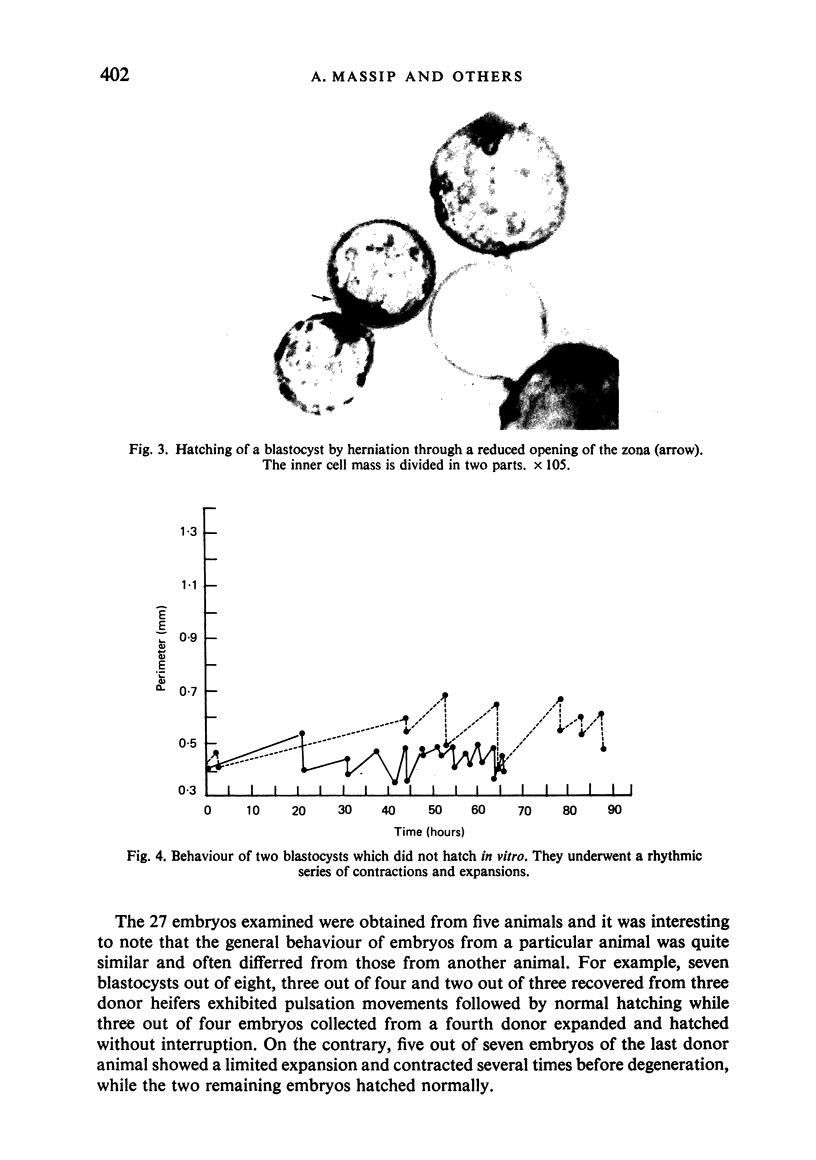



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Biggers J. D., Leonov B. V., Baskar J. F., Fried J. Inhibition of hatching of mouse blastocysts in vitro by prostaglandin antagonists. Biol Reprod. 1978 Oct;19(3):519–533. doi: 10.1095/biolreprod19.3.519. [DOI] [PubMed] [Google Scholar]
- Bin L., Mulnard J. Analyse cinématographique et morphométrique du comportement in vitro du blastocyste de la souris. Arch Biol (Liege) 1980;91(1):37–48. [PubMed] [Google Scholar]
- Bitton-Casimiri V., Brun J. L., Psychoyos A. Comportement in vitro des blastocystes du 5e jour de la gestation chez la ratte; étude micro-cinématographique. C R Acad Sci Hebd Seances Acad Sci D. 1970 Jun 15;270(24):2979–2982. [PubMed] [Google Scholar]
- Cole R. J. Cinemicrographic observations on the trophoblast and zona pellucida of the mouse blastocyst. J Embryol Exp Morphol. 1967 Jun;17(3):481–490. [PubMed] [Google Scholar]
- Ducibella T., Albertini D. F., Anderson E., Biggers J. D. The preimplantation mammalian embryo: characterization of intercellular junctions and their appearance during development. Dev Biol. 1975 Aug;45(2):231–250. doi: 10.1016/0012-1606(75)90063-9. [DOI] [PubMed] [Google Scholar]
- Fléchon J. E., Renard J. P. A scanning electron microscope study of the hatching of bovine blastocysts in vitro. J Reprod Fertil. 1978 May;53(1):9–12. doi: 10.1530/jrf.0.0530009. [DOI] [PubMed] [Google Scholar]
- Hastings R. A., 2nd, Enders A. C. Junctional complexes in the preimplantation rabbit embryo. Anat Rec. 1975 Jan;181(1):17–33. doi: 10.1002/ar.1091810103. [DOI] [PubMed] [Google Scholar]
- Lewis W. H., Gregory P. W. CINEMATOGRAPHS OF LIVING DEVELOPING RABBIT-EGGS. Science. 1929 Feb 22;69(1782):226–229. doi: 10.1126/science.69.1782.226-a. [DOI] [PubMed] [Google Scholar]
- Massip A., Mulnard J. Time-lapse cinematographic analysis of hatching of normal and frozen-thawed cow blastocysts. J Reprod Fertil. 1980 Mar;58(2):475–478. doi: 10.1530/jrf.0.0580475. [DOI] [PubMed] [Google Scholar]
- Mulnard J. G. Analyse microcinématographique du développement de l'oeuf de souris du stade II au blastocyste. Arch Biol (Liege) 1967;78(1):107–139. [PubMed] [Google Scholar]
- Newcomb R., Christie W. B., Rowson L. E. Non-surgical recovery of bovine embryos. Vet Rec. 1978 May 13;102(19):414–417. doi: 10.1136/vr.102.19.414. [DOI] [PubMed] [Google Scholar]
- Orsini M. W., McLaren A. Loss of the zona pellucida in mice, and the effect of tubal ligation and ovariectomy. J Reprod Fertil. 1967 Jun;13(3):485–499. doi: 10.1530/jrf.0.0130485. [DOI] [PubMed] [Google Scholar]
- Renard J. P., Philippon A., Menezo Y. In-vitro uptake of glucose by bovine blastocysts. J Reprod Fertil. 1980 Jan;58(1):161–164. doi: 10.1530/jrf.0.0580161. [DOI] [PubMed] [Google Scholar]
- Rowson L. E., Moor R. M., Lawson R. A. Fertility following egg transfer in the cow; effect of method, medium and synchronization of oestrus. J Reprod Fertil. 1969 Apr;18(3):517–523. doi: 10.1530/jrf.0.0180517. [DOI] [PubMed] [Google Scholar]
- Trounson A. O., Willadsen S. M., Rowson L. E. The influence of in-vitro culture and cooling on the survival and development of cow embryos. J Reprod Fertil. 1976 Jul;47(2):367–370. doi: 10.1530/jrf.0.0470367. [DOI] [PubMed] [Google Scholar]
- Wordinger R. J., Brinster R. L. Influence of reduced glucose levels on the in vitro hatching, attachment, and trophoblast outgrowth of the mouse blastocyst. Dev Biol. 1976 Oct 15;53(2):294–296. doi: 10.1016/0012-1606(76)90231-1. [DOI] [PubMed] [Google Scholar]