Abstract
1. Two fungi, Coniothyrium minitans Campbell and Trichoderma viride Pers. ex Fr., were grown on autoclaved crushed sclerotia of the species Sclerotinia sclerotiorum, which they parasitize. 2. In vitro the crude culture filtrates would lyse walls isolated from hyphal cells or the inner pseudoparenchymatous cells of the sclerotia, in which a branched β-(1→3)-β-(1→6)-glucan, sclerotan, is a major constituent. 3. Chromatographic fractionation of the enzymes in each culture filtrate revealed the presence of several laminarinases, the most active being an exo-β-(1→3)-glucanase, known from previous studies to attack sclerotan. Acting alone this brought about a limited degradation of the glucan, but the addition of fractions containing an endo-β-(1→3)-glucanase led to almost complete breakdown. A similar synergism between the two enzymes was found in their lytic action on cell walls. 4. When acting alone the endo-β-(1→3)-glucanase had a restricted action, the products including a trisaccharide, tentatively identified as 62-β-glucosyl-laminaribiose. 5. These results are discussed in relation to the structure of the cell walls and of their glucan constituents.
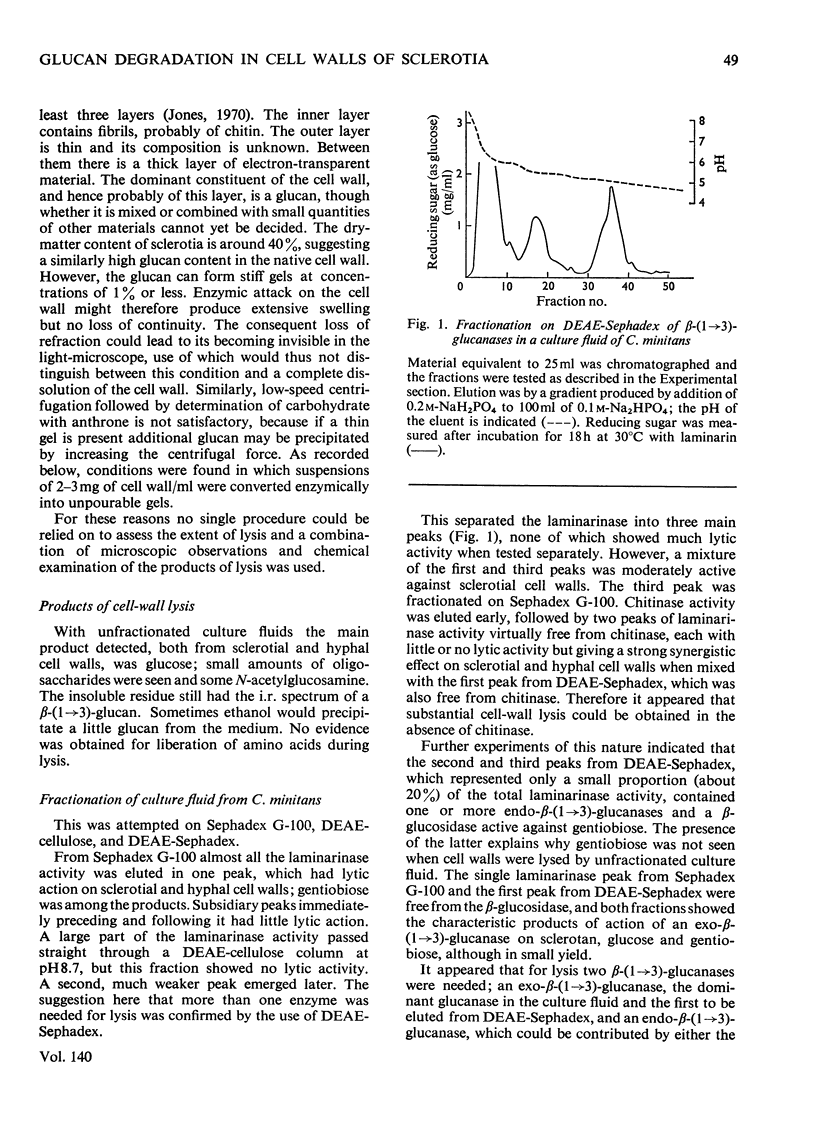
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bacon J. S., Farmer V. C., Jones D., Taylor I. F. The glucan components of the cell wall of baker's yeast (Saccharomyces cerevisiae) considered in relation to its ultrastructure. Biochem J. 1969 Sep;114(3):557–567. doi: 10.1042/bj1140557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacon J. S., Gordon A. H., Jones D., Taylor I. F., Webley D. M. The separation of beta-glucanases produced by Cytophaga johnsonii and their role in the lysis of yeast cell walls. Biochem J. 1970 Nov;120(1):67–78. doi: 10.1042/bj1200067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck K. W., Chen A. W., Dickerson A. G., Chain E. B. Formation and structure of extracellular glucans produced by Claviceps species. J Gen Microbiol. 1968 May;51(3):337–352. doi: 10.1099/00221287-51-3-337. [DOI] [PubMed] [Google Scholar]
- CHESTERS C. G., BULL A. T. The enzymic degradation of laminarin. 2. The multicomponent nature of fungal laminarinases. Biochem J. 1963 Jan;86:31–38. doi: 10.1042/bj0860031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chet I., Henis Y., Mitchell R. Chemical composition of hyphal and sclerotial walls of Sclerotium rolfsii Sacc. Can J Microbiol. 1967 Feb;13(2):137–141. doi: 10.1139/m67-019. [DOI] [PubMed] [Google Scholar]
- Doi K., Doi A., Fukui T. Joint action of two glucanases produced by Arthrobacter in spheroplast formation from baker's yeast. J Biochem. 1971 Oct;70(4):711–714. doi: 10.1093/oxfordjournals.jbchem.a129686. [DOI] [PubMed] [Google Scholar]
- Fleming M., Manners D. J., Masson A. J. The enzymic degradation of laminarin. Biochem J. 1967 Aug;104(2):32P–33P. [PMC free article] [PubMed] [Google Scholar]
- Hasegawa S., Nordin J. H. Enzymes that hydrolyze fungal cell wall polysaccharides. I. Purification and properties of an endo-alpha-D-(1-3)-glucanase from Trichoderma. J Biol Chem. 1969 Oct 25;244(20):5460–5470. [PubMed] [Google Scholar]
- Huotari F. I., Nelson T. E., Smith F., Kirkwood S. Purification of an exo-beta-D-(1 bonded to 3)-glucanase from Basidiomycete species QM 806. J Biol Chem. 1968 Mar 10;243(5):952–956. [PubMed] [Google Scholar]
- Rees D. A. Structure, conformation, and mechanism in the formation of polysaccharide gels and networks. Adv Carbohydr Chem Biochem. 1969;24:267–332. doi: 10.1016/s0065-2318(08)60352-2. [DOI] [PubMed] [Google Scholar]


