Abstract
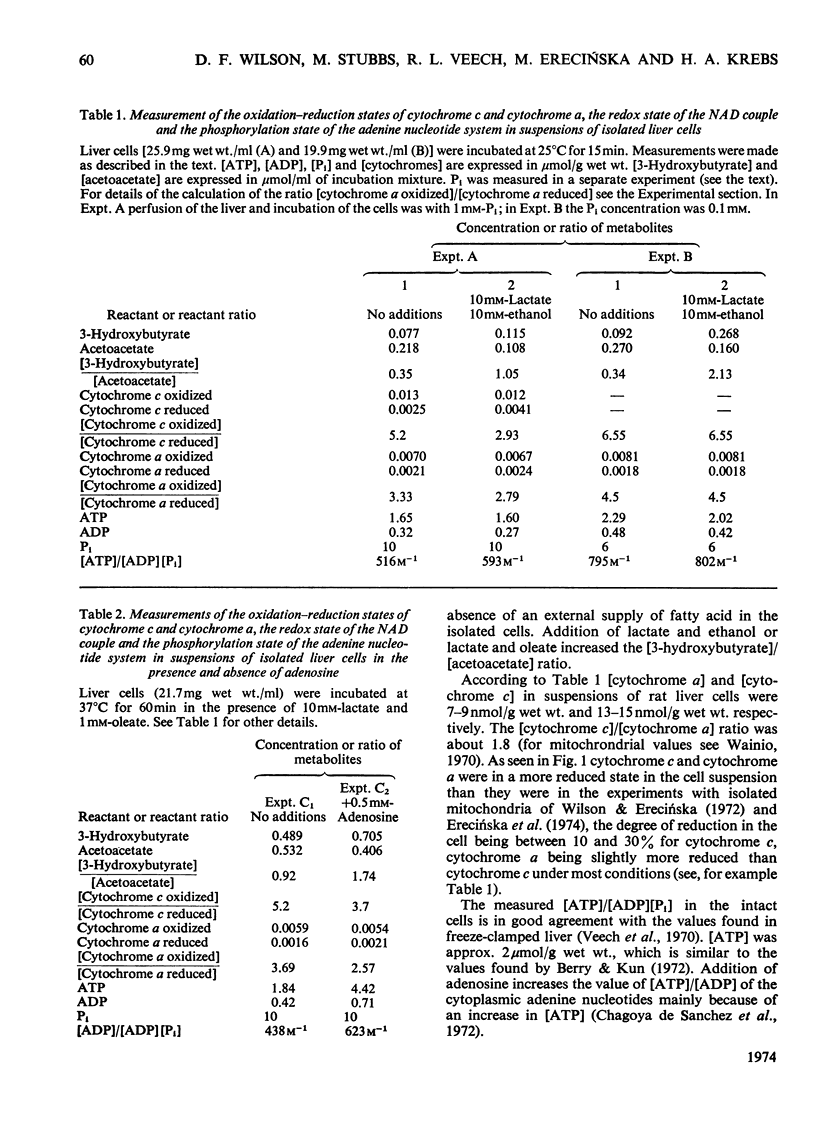
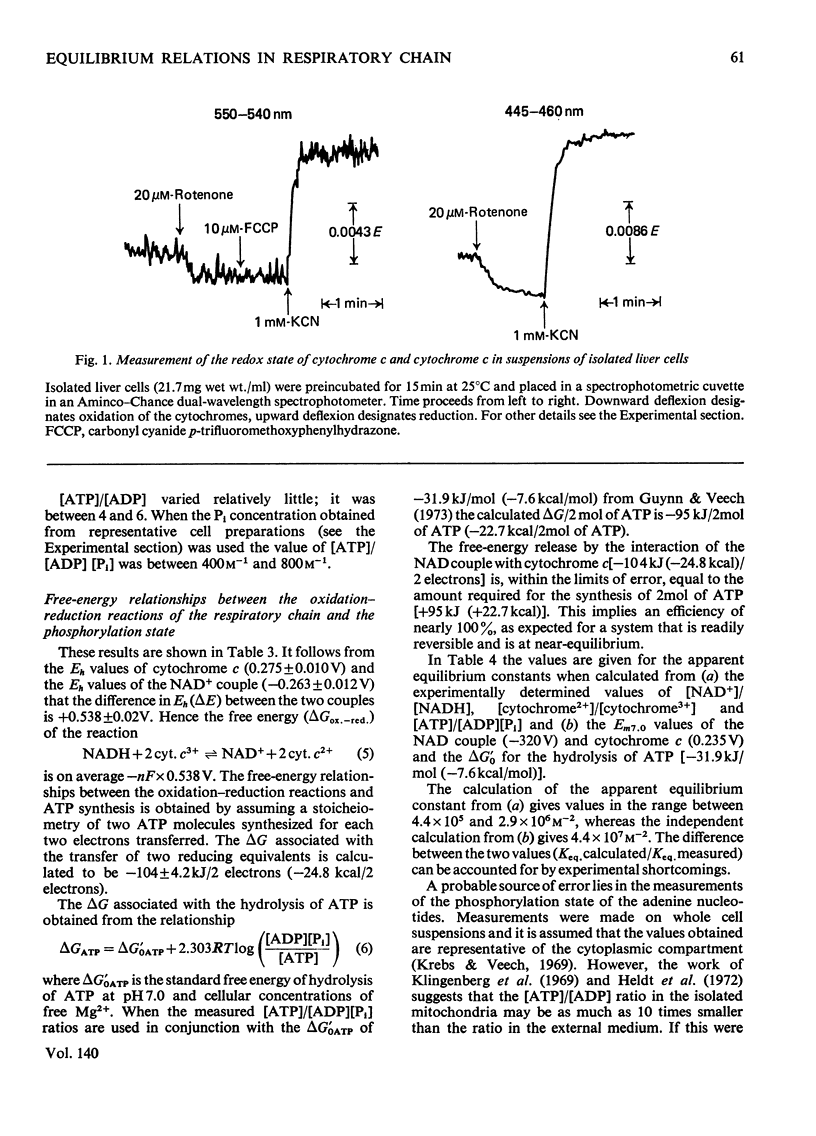
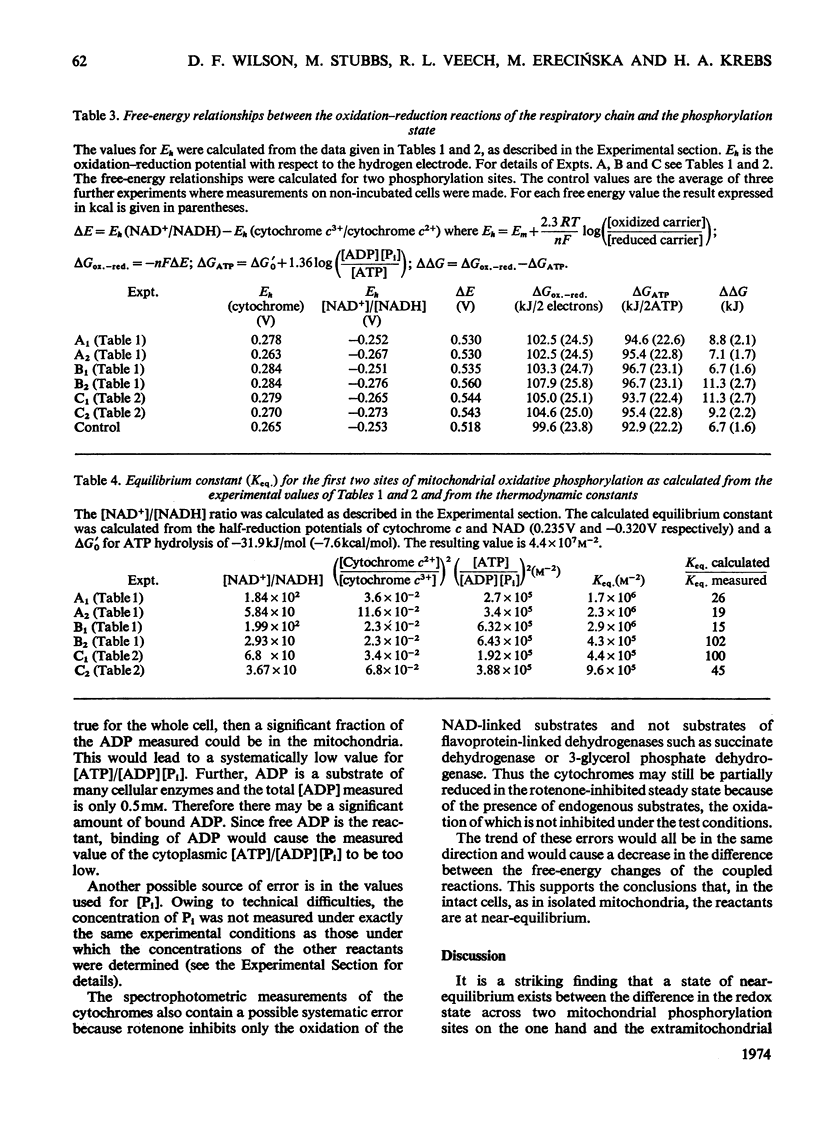
1. The redox state of cytochrome c, cytochrome a and the mitochondrial NAD couple, and the phosphorylation state of the adenine nucleotides, were measured in suspensions of isolated rat liver cells. 2. The ΔG for the transfer of two electrons from the mitochondrial NAD to the cytochrome c couple is calculated to be 104kJ (24.8kcal). 3. The ΔG associated with the synthesis of ATP at the measured phosphorylation state is calculated to be 95kJ (22.7kcal)/2mol of ATP. 4. The near equality of ΔG of the electron-transport process and ΔG required for ATP synthesis indicates near-equilibrium between the mitochondrial respiratory chain and the extramitochondrial phosphorylation state. 5. The existence of near-equilibrium in the coupled reactions implies that the respiratory activity depends on the ratio [ATP]/[ADP][Pi] and not on the concentrations of the individual reactants. 6. If the overall system of oxidative phosphorylation is at near-equilibrium, all intermediary reactions must also be at equilibrium. Hence if the intramitochondrial and extramitochondrial phosphorylation states are indeed different, it follows that any differences in the activities of ATP, ADP and Pi must be coupled to ion gradients and/or potentials across the inner mitochondrial membrane in such a way that translocation occurs without loss of free energy. 7. The metabolic state of the mitochondria in the cell can be defined by the turnover number of the cytochromes, the cytoplasmic phosphorylation state, and the oxidation–reduction potential of the NAD couple, rather than by the availability of ADP, substrate and O2.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berry M. N., Friend D. S. High-yield preparation of isolated rat liver parenchymal cells: a biochemical and fine structural study. J Cell Biol. 1969 Dec;43(3):506–520. doi: 10.1083/jcb.43.3.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry M. N., Kun E. Rate-limiting steps of gluconeogenesis in liver cells as determined with the aid of fluoro-dicarboxylic acids. Eur J Biochem. 1972 May 23;27(2):395–400. doi: 10.1111/j.1432-1033.1972.tb01850.x. [DOI] [PubMed] [Google Scholar]
- CHANCE B., HOLLUNGER G. The interaction of energy and electron transfer reactions in mitochondria. VI. The efficiency of the reaction. J Biol Chem. 1961 May;236:1577–1584. [PubMed] [Google Scholar]
- CHANCE B., WILLIAMS G. R. The respiratory chain and oxidative phosphorylation. Adv Enzymol Relat Subj Biochem. 1956;17:65–134. doi: 10.1002/9780470122624.ch2. [DOI] [PubMed] [Google Scholar]
- Chagoya de Sánchez V., Brunner A., Piña E. In vivo modification of the energy charge in the liver cell. Biochem Biophys Res Commun. 1972 Feb 16;46(3):1441–1445. doi: 10.1016/s0006-291x(72)80138-4. [DOI] [PubMed] [Google Scholar]
- Cockrell R. S., Harris E. J., Pressman B. C. Energetics of potassium transport in mitochondria induced by valinomycin. Biochemistry. 1966 Jul;5(7):2326–2335. doi: 10.1021/bi00871a022. [DOI] [PubMed] [Google Scholar]
- Cornell N. W., Lund P., Hems R., Krebs H. A. Acceleration of gluconeogenesis from lactate by lysine (Short Communication). Biochem J. 1973 Jun;134(2):671–672. doi: 10.1042/bj1340671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutton P. L., Wilson D. F., Lee C. P. Oxidation-reduction potentials of cytochromes in mitochondria. Biochemistry. 1970 Dec 22;9(26):5077–5082. doi: 10.1021/bi00828a006. [DOI] [PubMed] [Google Scholar]
- Guynn R. W., Veech R. L. The equilibrium constants of the adenosine triphosphate hydrolysis and the adenosine triphosphate-citrate lyase reactions. J Biol Chem. 1973 Oct 25;248(20):6966–6972. [PubMed] [Google Scholar]
- Heldt H. W., Klingenberg M., Milovancev M. Differences between the ATP-ADP ratios in the mitochondrial matrix and in the extramitochondrial space. Eur J Biochem. 1972 Nov 7;30(3):434–440. doi: 10.1111/j.1432-1033.1972.tb02115.x. [DOI] [PubMed] [Google Scholar]
- KLINGENBERG M., SCHOLLMEYER P. [On the reversibility of oxidative phosphorylation. III. Effect of adenosine triphosphate on the respiratory chain in respiratory inhibited mitochondria]. Biochem Z. 1961;335:243–262. [PubMed] [Google Scholar]
- KREBS H. A., MELLANBY J., WILLIAMSON D. H. The equilibrium constant of the beta-hydroxybutyric-dehydrogenase system. Biochem J. 1962 Jan;82:96–98. doi: 10.1042/bj0820096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klingenberg M. Metabolite transport in mitochondria: an example for intracellular membrane function. Essays Biochem. 1970;6:119–159. [PubMed] [Google Scholar]
- Krebs H. A., Wallace P. G., Hems R., Freedland R. A. Rates of ketone-body formation in the perfused rat liver. Biochem J. 1969 May;112(5):595–600. doi: 10.1042/bj1120595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsay J. G., Wilson D. F. Apparent adenosine triphosphate induced ligand change in cytochrome a 3 of pigeon heart mitochondria. Biochemistry. 1972 Nov 21;11(24):4613–4621. doi: 10.1021/bi00774a031. [DOI] [PubMed] [Google Scholar]
- RIESKE J. S., HANSEN R. E., ZAUGG W. S. STUDIES ON THE ELECTRON TRANSFER SYSTEM. 58. PROPERTIES OF A NEW OXIDATION-REDUCTION COMPONENT OF THE RESPIRATORY CHAIN AS STUDIED BY ELECTRON PARAMAGNETIC RESONANCE SPECTROSCOPY. J Biol Chem. 1964 Sep;239:3017–3022. [PubMed] [Google Scholar]
- Sato N., Wilson D. F., Chance B. The spectral properties of the b cytochromes in intact mitochondria. Biochim Biophys Acta. 1971 Nov 2;253(1):88–97. doi: 10.1016/0005-2728(71)90236-2. [DOI] [PubMed] [Google Scholar]
- Slater E. C., Rosing J., Mol A. The phosphorylation potential generated by respiring mitochondria. Biochim Biophys Acta. 1973 Apr 5;292(3):534–553. doi: 10.1016/0005-2728(73)90003-0. [DOI] [PubMed] [Google Scholar]
- Stubbs M., Veech R. L., Krebs H. A. Control of the redox state of the nicotinamide-adenine dinucleotide couple in rat liver cytoplasm. Biochem J. 1972 Jan;126(1):59–65. doi: 10.1042/bj1260059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veech R. L., Raijman L., Krebs H. A. Equilibrium relations between the cytoplasmic adenine nucleotide system and nicotinamide-adenine nucleotide system in rat liver. Biochem J. 1970 Apr;117(3):499–503. doi: 10.1042/bj1170499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILLIAMSON D. H., MELLANBY J., KREBS H. A. Enzymic determination of D(-)-beta-hydroxybutyric acid and acetoacetic acid in blood. Biochem J. 1962 Jan;82:90–96. doi: 10.1042/bj0820090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson D. H., Lund P., Krebs H. A. The redox state of free nicotinamide-adenine dinucleotide in the cytoplasm and mitochondria of rat liver. Biochem J. 1967 May;103(2):514–527. doi: 10.1042/bj1030514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson D. F., Brocklehurst E. S. Energy dependent changes in the cytochromes of the mitochondrial respiratory chain. Arch Biochem Biophys. 1973 Sep;158(1):200–212. doi: 10.1016/0003-9861(73)90614-0. [DOI] [PubMed] [Google Scholar]
- Wilson D. F., Owen C., Mela L., Weiner L. Control of mitochondrial respiration by the phosphate potential. Biochem Biophys Res Commun. 1973 Jul 2;53(1):326–333. doi: 10.1016/0006-291x(73)91437-x. [DOI] [PubMed] [Google Scholar]


