Abstract
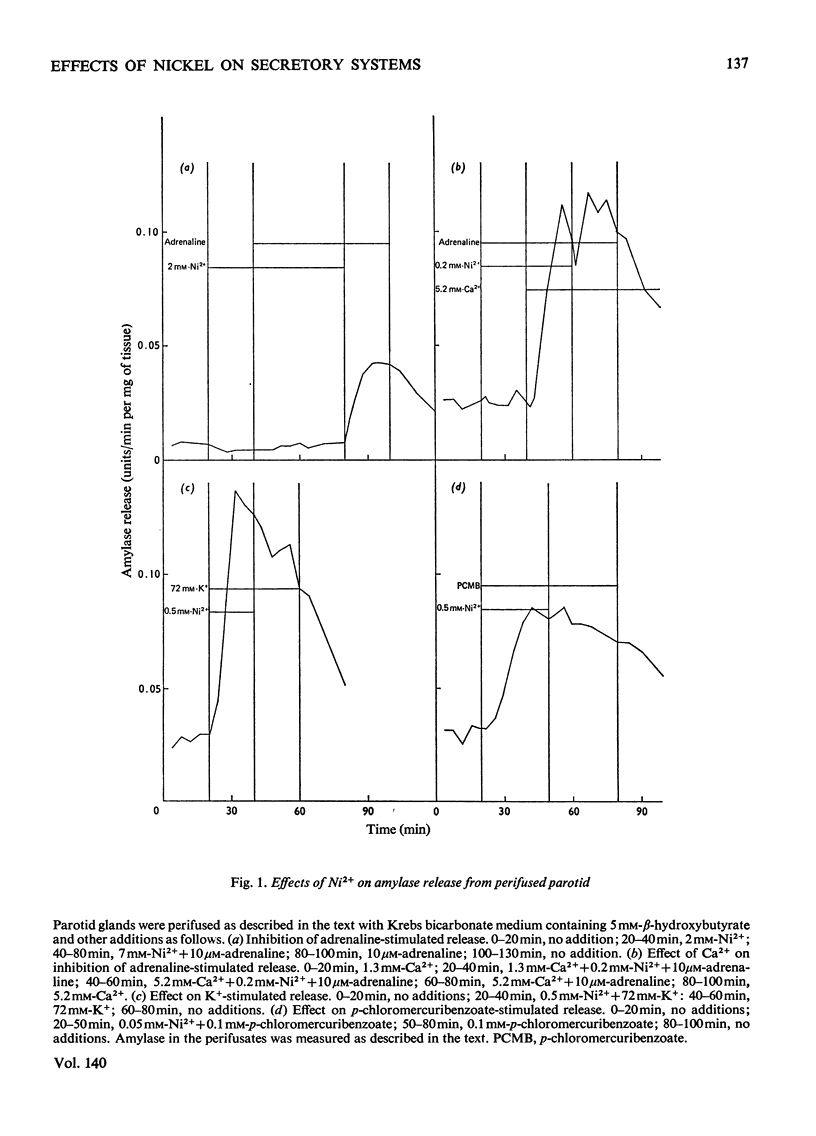
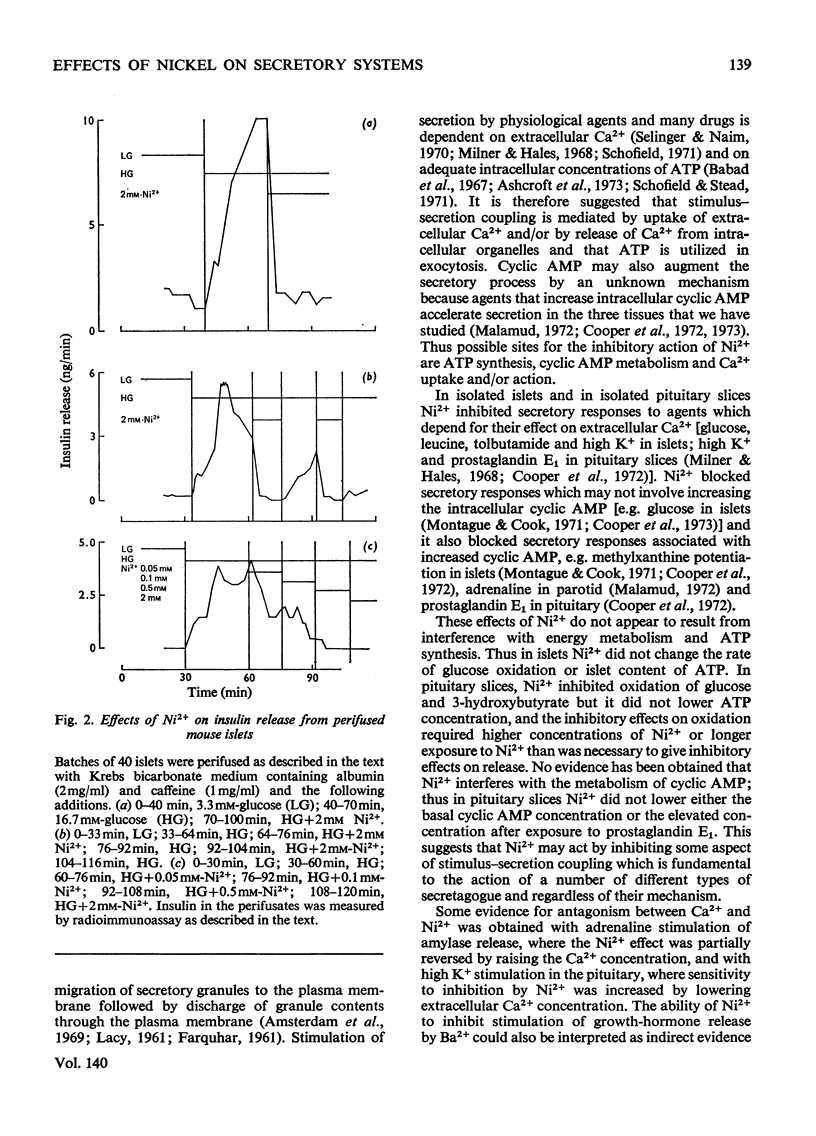
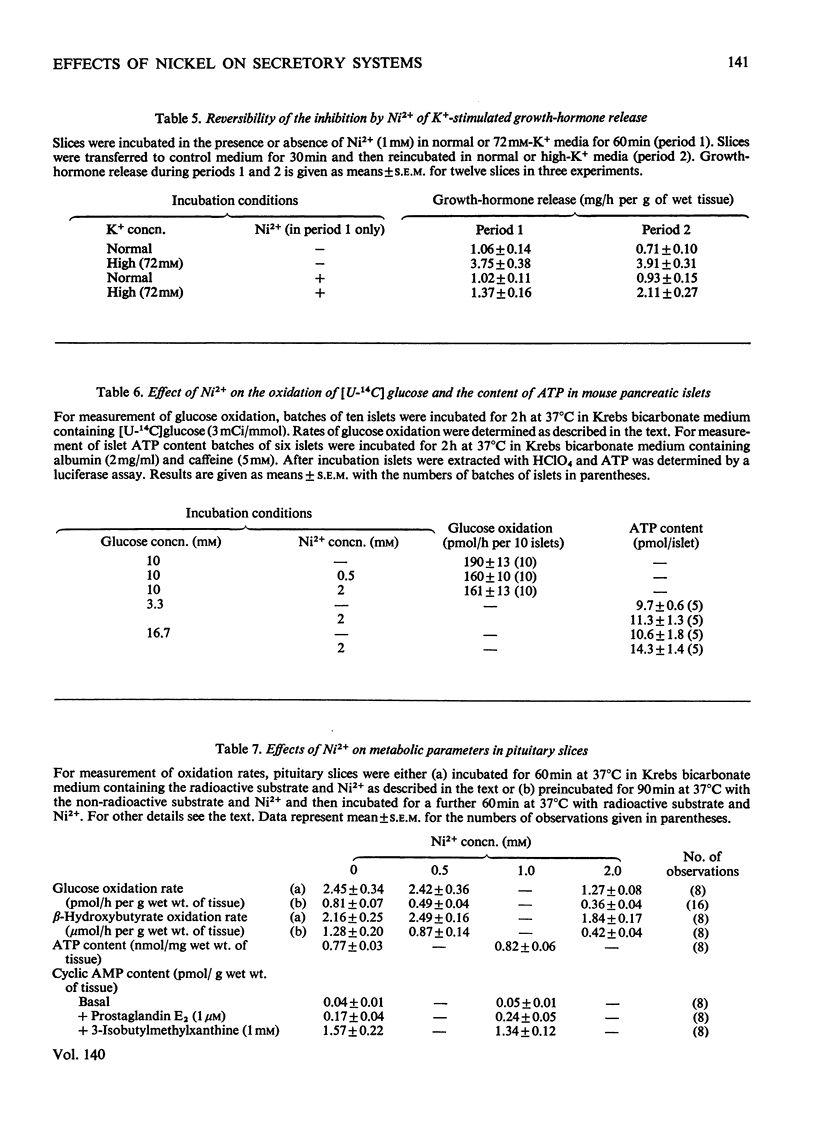
The effects of Ni2+ on the release of amylase from rat parotids, insulin from mouse pancreatic islets and growth hormone from bovine pituitary slices were investigated. In all these secretory systems, Ni2+ was shown to inhibit release evoked by a variety of stimuli both physiological and pharmacological. Measurements of rates of substrate oxidation and tissue concentrations of ATP and 3′:5′-cyclic AMP suggest that this inhibitory action of Ni2+ does not arise through an effect on energy metabolism or cyclic AMP metabolism. It is concluded that although some effects of Ni2+ may involve antagonism between Ni2+ and Ca2+ in stimulus–secretion coupling, others appear to be independent of Ca2+. It is suggested that Ni2+ may block exocytosis by interfering with either secretory-granule migration or membrane fusion and microvillus formation. The possible mode of action of Ni2+ and its potential use as a tool in the study of exocytosis are discussed.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amsterdam A., Ohad I., Schramm M. Dynamic changes in the ultrastructure of the acinar cell of the rat parotid gland during the secretory cycle. J Cell Biol. 1969 Jun;41(3):753–773. doi: 10.1083/jcb.41.3.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashcroft S. J., Weerasinghe L. C., Randle P. J. Interrelationship of islet metabolism, adenosine triphosphate content and insulin release. Biochem J. 1973 Feb;132(2):223–231. doi: 10.1042/bj1320223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babad H., Ben-Zvi R., Bdolah A., Schramm M. The mechanism of enzyme secretion by the cell. 4. Effects of inducers, substrates and inhibitors on amylase secretion by rat parotid slices. Eur J Biochem. 1967 Mar;1(1):96–101. doi: 10.1111/j.1432-1033.1967.tb00049.x. [DOI] [PubMed] [Google Scholar]
- Benoit P. R., Mambrini J. Modification of transmitter release by ions which prolong the presynaptic action potential. J Physiol. 1970 Oct;210(3):681–695. doi: 10.1113/jphysiol.1970.sp009235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coll-Garcia E., Gill J. R. Insulin release by isolated pancreatic islets of the mouse incubated in vitro. Diabetologia. 1969 Apr;5(2):61–66. doi: 10.1007/BF01211999. [DOI] [PubMed] [Google Scholar]
- Cooper R. H., Ashcroft S. J., Randle P. J. Concentration of adenosine 3':5'-cyclic monophosphate in mouse pancreatic islets measured by a protein-binding radioassay. Biochem J. 1973 Jun;134(2):599–605. doi: 10.1042/bj1340599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper R. H., McPherson M., Schofield J. G. The effect of prostaglandins on ox pituitary content of adenosine 3':5'-cyclic monophosphate and the release of growth hormone. Biochem J. 1972 Mar;127(1):143–154. doi: 10.1042/bj1270143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas W. W. Stimulus-secretion coupling: the concept and clues from chromaffin and other cells. Br J Pharmacol. 1968 Nov;34(3):451–474. doi: 10.1111/j.1476-5381.1968.tb08474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FARQUHAR M. G. Origin and fate of secretory granules in cells of the anterior pituitary gland. Trans N Y Acad Sci. 1961 Feb;23:346–351. doi: 10.1111/j.2164-0947.1961.tb01361.x. [DOI] [PubMed] [Google Scholar]
- KAUFMANN R., FLECKENSTEIN A. CA++-KOMPETITIVE ELEKTRO-MECHANISCHE ENTKOPPELUNG DURCH NI++- UND CO++-IONEN AM WARMBLUETERMYODARD. Pflugers Arch Gesamte Physiol Menschen Tiere. 1965;282:290–297. [PubMed] [Google Scholar]
- Kleinfeld M., Stein E. Action of divalent cations on membrane potentials and contractility in rat atrium. Am J Physiol. 1968 Sep;215(3):593–599. doi: 10.1152/ajplegacy.1968.215.3.593. [DOI] [PubMed] [Google Scholar]
- LACY P. E. Electron microscopy of the beta cell of the pancreas. Am J Med. 1961 Dec;31:851–859. doi: 10.1016/0002-9343(61)90024-9. [DOI] [PubMed] [Google Scholar]
- LaBella F., Dular R., Vivian S., Queen G. Pituitary hormone releasing or inhibiting activity of metal ions present in hypothalamic extracts. Biochem Biophys Res Commun. 1973 Jun 8;52(3):786–791. doi: 10.1016/0006-291x(73)91006-1. [DOI] [PubMed] [Google Scholar]
- Malamud D. Amylase secretion from mouse parotid and pancreas: role of cyclic AMP and isoproterenol. Biochim Biophys Acta. 1972 Sep 15;279(2):373–376. doi: 10.1016/0304-4165(72)90155-9. [DOI] [PubMed] [Google Scholar]
- McPherson M. A., Schofield J. G. Cytochalasin B, pituitary metabolism, and the release of ox growth hormone in vitro. FEBS Lett. 1972 Jul 15;24(1):45–48. doi: 10.1016/0014-5793(72)80822-6. [DOI] [PubMed] [Google Scholar]
- Meiri U., Rahamimoff R. Neuromuscular transmission: inhibition by manganese ions. Science. 1972 Apr 21;176(4032):308–309. doi: 10.1126/science.176.4032.308. [DOI] [PubMed] [Google Scholar]
- Milner R. D., Hales C. N. Cations and the secretion of insulin. Biochim Biophys Acta. 1968 Jan 3;150(1):165–167. doi: 10.1016/0005-2736(68)90022-9. [DOI] [PubMed] [Google Scholar]
- Montague W., Cook J. R. The role of adenosine 3':5'-cyclic monophosphate in the regulation of insulin release by isolated rat islets of Langerhans. Biochem J. 1971 Mar;122(1):115–120. doi: 10.1042/bj1220115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robberecht P., Christophe J. Secretion of hydrolases by perfused fragments of rat pancreas: effect of calcium. Am J Physiol. 1971 Apr;220(4):911–917. doi: 10.1152/ajplegacy.1971.220.4.911. [DOI] [PubMed] [Google Scholar]
- Schofield J. G. Effect of sulphydryl reagents on the release of ox growth hormone in vitro. Biochim Biophys Acta. 1971 Dec 21;252(3):516–525. doi: 10.1016/0304-4165(71)90154-1. [DOI] [PubMed] [Google Scholar]
- Schofield J. G. Measurement of growth hormone released by ox anterior-pituitary slices in vitro. Biochem J. 1967 May;103(2):331–341. doi: 10.1042/bj1030331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schofield J. G., Stead Margaret. ATP, calcium uptake and growth hormone release. FEBS Lett. 1971 Mar 5;13(3):149–151. doi: 10.1016/0014-5793(71)80222-3. [DOI] [PubMed] [Google Scholar]
- Selinger Z., Naim E. The effect of calcium on amylase secretion by rat parotid slices. Biochim Biophys Acta. 1970 Apr 21;203(2):335–337. doi: 10.1016/0005-2736(70)90148-3. [DOI] [PubMed] [Google Scholar]



