Abstract
The Italian Carciofo di Paestum (C. scolymus) PGI, an artichoke variety from the Campania region, was investigated for its potential to reuse by-products for food supplements. EtOH:H2O 50:50 and 75:25 extracts of its leaves were analyzed for phenolic and flavonoid content and antioxidant activity (TEAC: 1.90 and 1.81 mM of Trolox; DPPH IC50: 106.31 µg/mL and 128.21 µg/mL; FRAP: 1.68 and 1.58 mM FeSO₄/g extract). To further investigate the antioxidant potential, the ability of the two extracts to scavenge reactive species was assessed in Caco-2 cell cultures, showing a dose-dependent antioxidant capacity. To highlight metabolites responsible for the activity, LC-ESI/HRMSMS analysis was achieved, revealing 28 compounds (sesquiterpenes, megastigmanes, quinic acid and hydroxycinnamic acid derivatives, flavonoids, lignans, triterpenoid saponins, and polar fatty acids), of which structures were determined using 1D- and 2D-NMR analysis. In addition, quantitative determination of caffeoyl, dicaffeoyl, and quinic acid derivatives (CQAs) was performed through LC-ESI/QTrap/MS/MS, highlighting that the most abundant compound was 5-caffeoylquinic acid (6), with values of 9.310 and 7.603 mg/g extract in EtOH:H2O (75:25) and EtOH:H2O (50:50), respectively. The analysis showed that extracts were rich in bioactive compounds, suggesting their potential for development into antioxidant-based food supplements that may protect cells from oxidative stress and support overall wellness.
Keywords: “Carciofo di Paestum” PGI leaves, eco-sustainable extracts, antioxidant activity on Caco-2 cells, LC-ESI/HRMS analysis, NMR analysis, specialized metabolites
1. Introduction
The globe artichoke, scientifically known as Cynara cardunculus var. scolymus, belongs to the Asteraceae family. It is a perennial plant highly valued for its edible immature flower buds, which are harvested before they fully bloom [1,2,3]. Flower buds are commonly referred to as “capitula” or “heads” and represent the edible part; they are consumed fresh, canned, or frozen [4,5,6].
Italy is a global leader in artichoke production, accounting for 390 Kt (26%) of the total worldwide production [7,8]. One of the most well-known cultivars of artichokes in Italy is “Carciofo di Paestum” PGI (Protected Geographical Indication), which customers and chefs prize for its unique flavor, delicacy, and size [9]. The main growing area for the above-mentioned cultivar is Paestum, a town in the Campania region, Southern Italy, known for its bountiful plains and rich agricultural heritage. The excellent taste and quality of this particular variety of artichoke are the result of the region’s special soil and environment [9].
External bracts and leaves, as well as stems, stalks, roots, and, to a lesser extent, seeds, represent by-products of industrial processing [10]. According to FAO estimates, 1.3 billion tons of edible food are lost or wasted annually, producing about 190,000 tons of by-products, with a significant financial impact on the global economy (USD 750 billion annually) [7]. The United Nations continues to promote recovery policies and circular economy strategies to reduce waste and encourage reuse, as the environmental and economic impacts of food waste disposal are growing concerns. Among artichoke by-products, residual leaves are rich in nutrients (carbohydrates and proteins) and bioactive compounds, which represent potential ingredients for foodstuffs, functional foods, and food supplements due to their functional and biological properties. The main specialized metabolites reported in artichoke leaves are flavonoids, sesquiterpenes, and caffeoyl, dicaffeoyl, and quinic acid derivatives (CQAs) [11,12]. These compounds are a subgroup of phenolic acids composed of quinic acid linked to one to four caffeoyl units. They are known for their hepatoprotective properties and potent antioxidant activity, which help scavenge free radicals and protect cells from oxidative damage [13,14,15].
Supported by these premises, for the first time, eco-sustainable extracts of “Carciofo di Paestum” PGI leaves were investigated in order to add value to artichoke leaf by-products as a source of bioactive phytochemicals. Food supplements are typically extracted using non-toxic solvents, such as ethanol and water. Ethanol, a widely used solvent, is biodegradable [16]. As a result, eco-friendly solvents, including water and aqueous ethanol solutions, are favored in extraction processes [17,18]; therefore, herein, two different eco-friendly mixtures were used.
In detail, two eco-sustainable EtOH:H2O (50:50 and 75:25) extracts of artichoke leaves have been investigated for the total phenolic and flavonoid content and the radical scavenging activity through 1,1-diphenyl-2-picrylhydrazyl (DPPH), Trolox Equivalent Antioxidant Capacity (TEAC), and ferric reducing/antioxidant power (FRAP) assays. Furthermore, a cell-based antioxidant in vitro test was also conducted on differentiated intestinal Caco-2 cells. In order to identify the compounds mainly responsible for the antioxidant activity, a phytochemical analysis was performed on both extracts. In the first step, eco-sustainable extracts were analyzed through liquid chromatography coupled to electrospray ionization and high-resolution mass spectrometry (LC–ESI/HRMS) in negative ion mode.
To assign the chemical structures of the compounds detected through the LC-ESI/HRMS analysis, a phytochemical analysis of the EtOH:H2O 50:50 extract was conducted, leading to the isolation and structural identification of the metabolites through 1D- and 2D-NMR experiments. Finally, considering the bioactivities reported for caffeoyl, dicaffeoyl, quinic acid derivatives (CQAs) [19], quantitative analysis through liquid chromatography coupled with tandem mass spectrometry using an ESI source and a hybrid triple quadrupole-linear ion trap mass analyzer (LC-ESI/QTrap/MS/MS) was performed.
2. Results and Discussion
2.1. Extraction of the “Carciofo di Paestum” PGI Leaves and Evaluation of Phenolic and Flavonoid Content and Antioxidant Activity by Spectrophotometric Assays
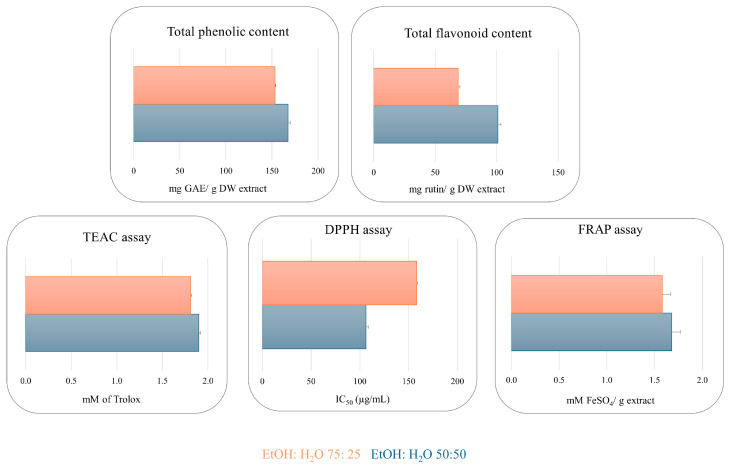
The Folin–Ciocalteu assay was employed to determine the phenolic content. EtOH:H2O 50:50 and 75:25 extracts displayed a high concentration of phenolic compounds with a value of 167.48 and 153.41 (mg GAE/g extract), respectively. The same trend has been observed for the flavonoid content, with a value of 101.02 and 67.49 mg rutin/g extract value. A TEAC assay was performed successfully to obtain preliminary information about the radical scavenging properties of the extracts [20]. The extracts showed interesting antioxidant activity with a value of 1.90 and 1.81 (mM of Trolox) for EtOH:H2O 50:50 and EtOH:H2O 75:25, respectively, which is comparable to quercetin used as a reference compound (2.51 mM). The DPPH and FRAP assays displayed the same trend shown by the TEAC assay, with IC50 = 106.31 µg/mL and 128.21 µg/mL for DPPH and 1.68 and 1.58 mM FeSO4/g extract for FRAP assay (Figure 1 and Table S1). The remarkable antioxidant results, determined through spectrophotometric assays, confirm that flavonoids and phenolic derivatives contribute to the radical scavenging activity of the extracts (Figure 1 and Table S1) [21].
Figure 1.
Total phenolic and flavonoid content, TEAC, DPPH, and FRAP assays of EtOH:H2O (50:50) (blue) and EtOH:H2O (75:25) (orange) extracts of “Carciofo di Paestum” PGI leaves.
2.2. Cytotoxicity Evaluation in Caco-2 Cells Monolayers
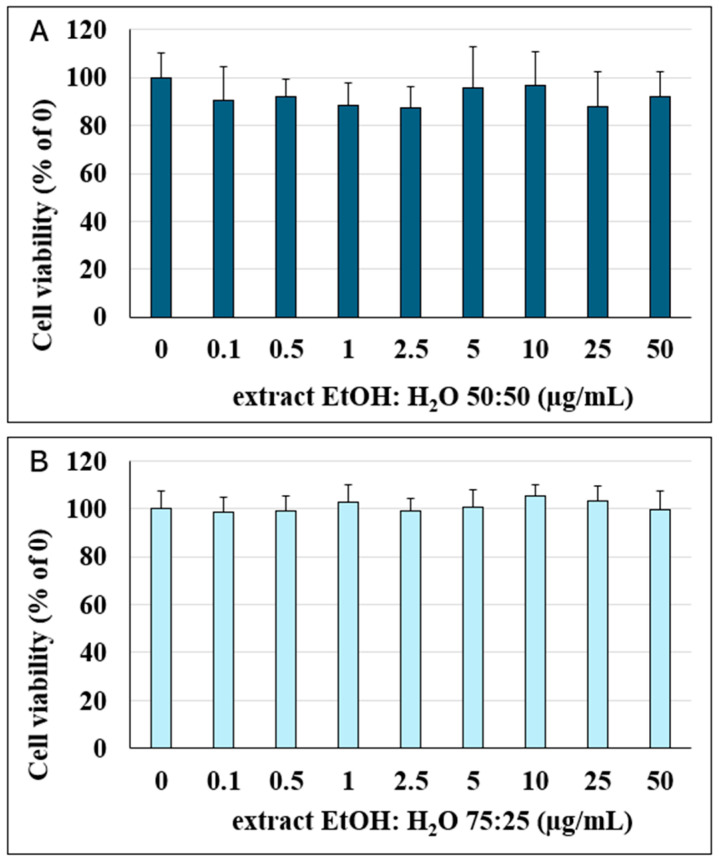
Preliminarily, before evaluating the antioxidant capacity of the extracts on Caco-2 cells’ monolayers, cell viability was first assessed following treatment with different concentrations of the extracts. As shown in Figure 2, cell viability was not significantly affected by either extract at any of the concentrations tested (0.1–50 μM) after 24 h of incubation. Therefore, different concentrations of the extracts were tested to evaluate their antioxidant activity.
Figure 2.
Percentage of viable Caco-2 cells calculated relative to the control (0 μM, 100% viability) following 24 h of incubation with different concentrations of EtOH:H2O (50:50) (A) and EtOH:H2O (75:25) (B) extracts (0.1–50 μM). Data are presented as the mean ± SD from independent experiments (n = 16).
2.3. Antioxidant Activity Against TBH-Induced Oxidative Stress in Caco-2 Cells’ Monolayers
With the aim to confirm the antioxidant activity observed through spectrophotometric assays, an in vitro antioxidant test was conducted. Specifically, the capacity of the two extracts to scavenge reactive species was assessed in Caco-2 cell cultures by measuring intracellular ROS levels using 2′,7′-DCFH-DA.
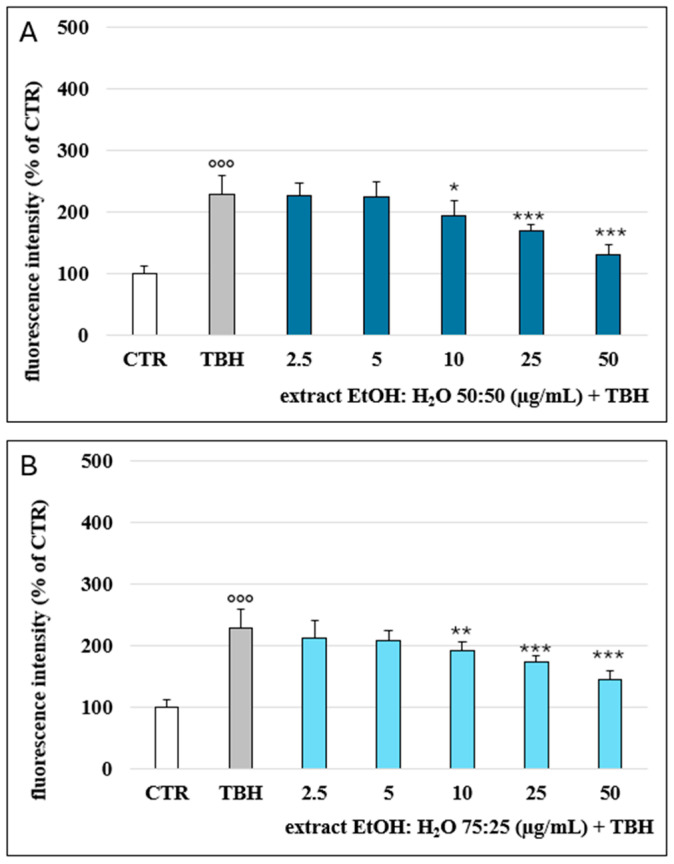
Treatment with tert-butylhydroperoxide (TBH) (2.5 mM) resulted in a significant increase in ROS production (120% ROS increase at 2 h of incubation) in comparison with the non-treated cells (CTR), as reported in Figure 3. The pretreatment with the two extracts partially counteracted TBH-induced oxidative stress in a dose-dependent manner. The extracts, tested at 2.5 and 5 μg/mL, did not affect the oxidative stress but significantly inhibited ROS production at 10, 25, and 50 μg/mL. Substantially, no difference was noted between the two extracts tested, except for the EtOH:H2O (75:25) extract, tested at 10 μg/mL, which showed a higher significance (p < 0.01) than the EtOH:H2O (50:50) extract (p < 0.05). These data confirm the biological action of artichoke phenols as previously reported, where phenolic extracts of different parts of the artichoke were found to be active against oxidative stress and inflammation in the same cellular model [22,23,24].
Figure 3.
ROS levels, detected via H2-DCF-DA fluorescence and expressed as % of the control samples (CTR) in Caco-2 cells following 2 h of incubation with varying concentrations of the EtOH:H2O (50:50) (A) and EtOH:H2O (75:25) (B) (2.5–50 μM) in co-incubation with TBH 2.5 mM. °°° = p < 0.001 TBH vs. CTR; * = p < 0.05 extracts vs. TBH; ** = p < 0.01 extracts vs. TBH; *** = p < 0.001 extracts vs. TBH (n = 12).
2.4. LCESI/LTQOrbitrap/MS Analysis of “Carciofo di Paestum” Leaves’ Extracts
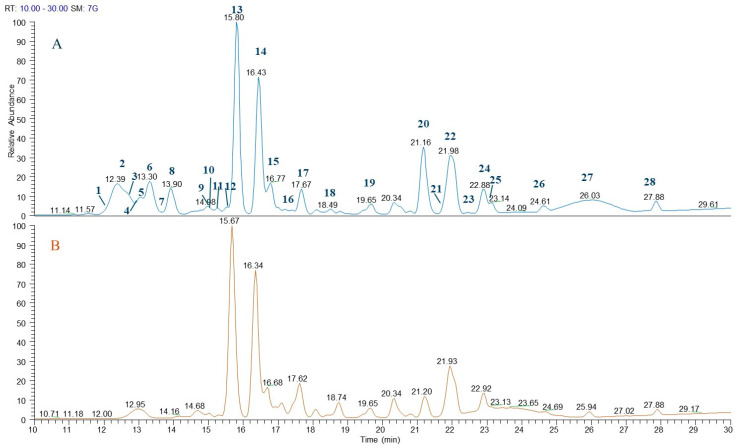
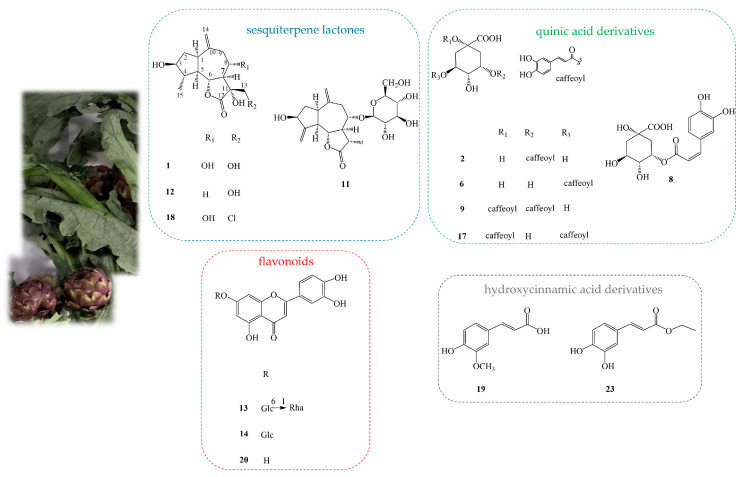
In order to identify bioactive metabolites in the extracts of artichoke leaves, LC-ESI/HRMS was achieved. A detailed analysis of the LC-ESI/HRMS spectra led to the identification of 28 compounds belonging to the class of sesquiterpenes (1, 3, 7, 11, 12, 18), megastigmanes (4, 15), quinic acid derivatives (2, 6, 8, 9, 10, 17), flavonoids (13, 14, 20), hydroxycinnamic acid derivatives (5, 19, 23), lignan (16), triterpenoid saponins (21, 25), and polar fatty acids (22, 24, 26–28) (Figure 4 and Table 1).
Figure 4.
LC-ESI/HRMS profile of EtOH:H2O (50:50) (A) and EtOH:H2O (75:25) (B) extracts of “Carciofo di Paestum” PGI leaves.
Table 1.
Metabolites occurring in “Carciofo di Paestum” PGI leaves’ extracts based on LC-ESI/HRMS.
| n° | Compound | Rt * (Min) | Molecular Formula | [M-H]− | [(M + HCOOH)-H]− | Δ ppm | Characteristic Product Ions |
|---|---|---|---|---|---|---|---|
| 1 | 3β, 8α, 11α, 13-tetrahydroxy-10 (14)-guaien-1α, 4β, 5α, 6βH-6α, 12-olide | 12.09 | C15H22O6 | 343.1396 | 2.66 | 297.1339 (C15H21O6) | |
| 2 | 3-caffeoyl quinic acid | 12.35 | C16H18O9 | 353.0874 | 1.00 | 191.0559 (C7H11O6), 179.0348 (C9H7O4), 173.0348 (C7H9O5), 135.0452 (C8H7O2) |
|
| 3 | Cynarascoloside A/B | 12.91 | C21H32O9 | 473.2024 | 0.68 | 427.2024 (C21H31O9), 179.0558 (C6H11O6) | |
| 4 | Megastigmane-O-hexoside | 13.00 | C19H32O8 | 433.2074 | 1.40 | 387.2016 (C18H29O6); 225.1003 (C13H21O3) | |
| 5 | Eugenol-O-rutinoside | 13.12 | C22H32O11 | 471.1869 | 1.64 | 163.1105 (C10H11O2) | |
| 6 | 5-caffeoyl quinic acid (chlorogenic acid) | 13.30 | C16H18O9 | 353.0876 | 2.38 | 191.0562 (C7H11O6), 179.0313 (C9H7O4), | |
| 7 | Cynarascoloside A/B | 13.80 | C21H32O9 | 473.2025 | 1.56 | 427.2025 (C21H31O9), 179.0558 (C6H11O6) | |
| 8 | 5-p-coumaroyl quinic acid | 13.90 | C16H18O8 | 337.0922 | 1.17 | 191.0561 (C7H11O6), 163.0452 (C9H7O3) | |
| 9 | 1, 3-dicaffeoyl quinic acid | 14.94 | C25H24O12 | 515.1190 | 1.08 | 353.0873 (C16H17O9), 191.0567 (C7H11O6), 179.0375 (C9H7O4) |
|
| 10 | Feruloyl quinic acid | 15.00 | C17H20O9 | 367.1030 | 1.75 | 193.0602 (C10H9O4), 191.0561 (C7H11O6) | |
| 11 | dodesacylcynaropicrin 8-O-β-D-glucopyranoside | 15.07 | C21H30O9 | 471.1865 | 0.92 | 425.1865 (C21H29O9), 179.0556 (C6H11O6) | |
| 12 | cynaratriol | 15.50 | C15H22O5 | 327.1445 | 2.05 | 263.1300 (C15H19O4) | |
| 13 | Luteolin 7-O-rutinoside | 15.80 | C27H30O15 | 593.1502 | 0.19 | 447.0923 (C21H19O11), 285.0400 (C15H9O6) | |
| 14 | Luteolin 7-O-β-D-glucopyranoside | 16.43 | C21H20O11 | 447.0930 | 1.79 | 285.0401 (C15H9O6) | |
| 15 | Megastigmandienone-O-hexoside | 16.77 | C19H30O7 | 415.1966 | 0.72 | 369.1916 (C18H27O5) 207.0586 (C13H19O2) | |
| 16 | Pinoresinol-O-hexoside | 16.77 | C26H32O11 | 519.1864 | 0.60 | 357.1339 (C20H21O6) | |
| 17 | 1, 5-dicaffeoyl quinic acid | 17.63 | C25H24O12 | 515.1185 | 0.31 | 353.0873 (C16H17O9), 191.0558 (C7H11O6), 179.0455 (C9H7O4) |
|
| 18 | Cynarinin B | 18.49 | C15H21O5Cl | 361.1052 | 1.05 | 315.0567 C15H20O5Cl | |
| 19 | Ferulic acid | 19.65 | C10H10O4 | 193.0507 | 6.24 | 161.0242 (C9H5O3) | |
| 20 | Luteolin | 21.16 | C15H10O6 | 285.0318 | 1.39 | 241.0503 (C14H9O4), 175.0398 (C10H7O3), 151.0037 (C7H3O4) |
|
| 21 | Cynarasaponin E | 21.29 | C42H66O15 | 809.4319 | 0.10 | 647.3795 (C36H55O10), 585.3792 (C35H53O7), 471.3471 (C30H47O4) |
|
| 22 | Trihydroxy octadecadienoic acid | 21.93 | C18H32O5 | 327.2171 | 1.53 | 229.1442 (C12H21O4) | |
| 23 | Caffeoyl-ethyl ester | 22.06 | C11H12O4 | 207.0663 | 3.91 | 179.0305 (C9H7O4) | |
| 24 | Trihydroxy octadecenoic acid | 22.88 | C18H34O5 | 329.2327 | 1.46 | 229.1441 (C12H21O4), 211.1338 (C12H19O3), 171.1022 (C9H15O3) |
|
| 25 | Cynarasaponin C | 23.14 | C42H66O14 | 839.4426 | 0.32 | 793.4374 (C42H65O14) 631.3845 (C36H55O9) | |
| 26 | Dihydroxy-octadecatrienoic acid | 24.60 | C18H30O4 | 309.2069 | 3.20 | 291.1960 (C18H27O3), 239.1646 (C14H23O3), 171.1022 (C9H15O3) |
|
| 27 | Hydroxy-oxooctadecatrienoic acid | 26.29 | C18H28O4 | 307.1911 | 2.42 | 289.1799 (C18H25O3), 235.1331 (C14H19O3) | |
| 28 | Hydroxy-octadecatrienoic acid | 27.88 | C17H26O4 | 293.1754 | 2.43 | 236.1049 (C13H16O4) |
Rt *: Retention time of the LC-ESI/HRMS profile of EtOH:H2O (50:50) extract.
Quinic acid derivatives and flavonoids represented the main classes of specialized metabolites. Compounds 2, 6, 8, 9, 10, and 17 showed a specific fragmentation at m/z 191 (C7H11O6) due to [quinic acid-H]−; therefore, these compounds were assigned as quinic acid derivatives. They also displayed fragments indicating the nature of the moiety linked to quinic acid; in particular, compounds 2 and 6 showed a fragment at m/z 179 (C9H7O4) corresponding to [caffeoyl-H]− and, consequently, they were assigned as caffeoyl–quinic acid derivatives; compounds 9 and 17 showed fragments at m/z 353 (C16H17O9) and m/z 179 (C9H7O4) related to [caffeoyl-quinic acid-H]− and [caffeoyl-H]−, respectively, and thus they were identified as dicaffeoyl–quinic acids. Finally, compounds 8 and 10 showed a fragment at m/z 163 (C9H7O3) and m/z 193 (C10H9O4) attributed to [cumaroyl-H]− and [feruloyl-H]−, respectively, linked to quinic acid [25]. Regarding flavonoid derivatives, the analysis of the fragmentation spectra of each compound allowed for the identification of the flavonoid class and the presence of one or more sugars linked to the aglycone. In detail, compounds 13 and 14, based on their fragmentation spectra, were assigned as luteolin 7-O-rutinoside and luteolin 7-O-β-D-glucopyranoside; according to the literature, both compounds displayed the base peak at m/z 285 corresponding to a molecular formula of C15H9O6 ascribable to [luteolin-H]−. Consequently, the loss of 308 Da and 162 Da corresponded to the dehydrated form of rutinoside and glucopyranoside [26,27]. Moreover, compound 20 was assigned as luteolin. All the above-mentioned compounds were previously reported in C. scolymus leaves [5,28] (Table 1).
By careful analysis of LC-ESI/HRMS spectra, compounds 1, 3, 7, 11, 12, and 18 were tentatively identified as sesquiterpene derivatives. Compounds 21 and 25 were assigned as cynarasaponin E and C, respectively, previously described in artichoke leaves extracts [28,29,30] (Table 1). Continuing the analysis of LC-ESI/HRMS profile, compounds 4 and 15 were tentatively assigned as megastigmane derivatives; in particular, after fragmentation, a base peak originated by neutral loss of dehydrated form of a hexose unit (162 Da) due to a deprotonated megastigmane unit was observed. Herein, megastigmane derivatives (4, 15) are reported for the first time in Cynara genus (Table 1). The LC-ESI/HRMS spectra of peak 16, after fragmentation, showed a product ion ascribable to the deprotonated phenolic compound generated by neutral loss of the dehydrated form of a hexose unit (neutral loss of 162 Da); in fact, peak 16 showed as main base peak m/z 357 (C20H21O6) corresponding to [pinoresionl-H]−. To the best of our knowledge, compound 16 is here reported for the first time in Cynara genus. Furthermore, the analysis of the exact mass and fragmentation spectra of compounds 5, 19, and 23 allowed us to assign them as hydroxycinnamic acid derivatives, in detail; they were identified as eugenol-O-rutinoside, ferulic acid, and caffeoyl ethyl ester, respectively. Among these compounds, 5 is reported for the first time in Cynara genus. Finally, peaks 22, 24, and 26–28 were designated as polar fatty acids belonging to oxylipin derivatives, as previously reported by Cerulli et al., 2021 [20] (Figure 4 and Table 1).
2.5. Isolation and Identification of Specialized Metabolites
In order to characterize unambiguously the main compounds in the extracts, EtOH:H2O (50:50) extract was submitted to Sephadex LH-20 and purified through HPLC-UV and HPLC-RI. In this way, compounds 1, 2, 6, 8, 9, 11–14, 17–20, and 23 were isolated, and their structures were determined through the combination of monodimensional and bidimensional NMR experiments and also comparing the results with the literature [9,31,32].
The 1H NMR spectrum of compound 1 showed the signals of three oxymethines at δ 3.64 (m), 4.08 (dd, J = 10.2, 4.2), and 4.16 (t, J = 10.3), an exomethylene at δ 5.12 (s), an oxymethylene at δ 3.77 (d, J = 10.2), and a signal at δ 1.23 (d, J = 6.0) ascribable to one methyl group (Table S2). In addition, the 13C NMR data of 1 displayed signals ascribable to a guaiane skeleton. The NMR data revealed the presence of 3β, 8α, 11α, 13- tetrahydroxy-10(14)-guaien-1α, 4β, 5α, 6βH-6α, and 12-olide (Table S2 and Figure 5). The 1H NMR spectra of 11, 12, and 18 (Table S2 and Figure 5) evidenced close similarities to compound 1, so 11, 12, and 18 were assigned to the same class but with slight differences. In particular, the 1H NMR spectrum of compound 2 differed from 1 for the absence of the oximethine function at C-8 (Table S2). The NMR data of compound 8 pointed to a close similarity of those of 1, with a likely replacement of the alcoholic group at C-13 of compound 1 with a chlorine atom in compound 18 (Table S2). The 1H NMR spectrum of compound 11 was superimposable to dodesacylcynaropicrin with additional signals ascribable to one hexose unit [30]; in particular, the careful analysis of 1D- and 2D-NMR spectra of 11 allowed us to assign a β-glucopyranosyl unit (δ 4.49) to C-8 (δ 84.8). In conclusion, compounds 11, 12, and 18 were assigned as dodesacyl-cynaropicrin 8-O-β-D-glucopyranoside, cynaratriol, and cynarinin B, respectively (Figure 5).
Figure 5.
Specialized metabolites isolated from EtOH:H2O (50:50) extract of “Carciofo di Paestum” PGI leaves.
The careful analysis of the NMR spectra of compounds 2 and 6 allowed us to identify single caffeoyl-substituted quinic acid derivatives. The 1H NMR spectra of compounds 2 and 6 showed signals for one caffeoyl unit in each compound at δ 7.58 and 7.55 (d, J = 16.0 Hz), δ 6.30 and 6.28 (d, J = 16.0 Hz), δ 7.07 and 7.06 (d, J = 1.8 Hz), δ 6.96 and 6.98 (dd, J = 1.8, 8.0), and δ 6.76 and 6.82 (d, J = 8.0 Hz), respectively, and signals ascribable to a quinic acid unit [33] (Table S3). The position of the caffeoyl substitution was determined by analyzing the chemical shifts and coupling constants of the oxygenated methine protons in the quinic acid core. Typically, the H-3 signal shows a small coupling constant and appears as a brd or br-type peak. In contrast, the H-5 signal appears as a ddd with key coupling constants (approximately 8.0, 8.0 and 3.0 Hz) [33] (Table S3, Figure 5). Due to caffeoyl acylation, the proton signal is down-field shifted [33]. Based on these rules, compounds 3 and 6 were assigned to 3-caffeoyl quinic acid, and 5-caffeoyl quinic acid, respectively (Figure 5).
The 1H NMR spectra of compounds 9 and 17 displayed similar signals to compounds 2 and 6, with an additional set of caffeoyl signals; therefore, compounds 9 and 17 were established as double caffeoyl-substituted quinic acid derivatives. Similarly to caffeoyl quinic acid derivatives, the acylation positions were determined by analyzing the chemical shifts and coupling constants of the oxygenated methine protons in the quinic acid core (Table S3 and Figure 5).
Compounds 13, 14, and 20 showed in 1D- and 2D-NMR spectra the typical chemical shifts of luteolin aglycone [9]; in addition, NMR experiments of compounds 13 and 14 exhibited, in the sugar region, signals of rutinoside and glucopyranoside moiety; consequently, the careful analysis of their NMR spectra allowed us to assign compounds 13, 14, and 20 as luteolin 7-O-rutinoside, luteolin 7-O-β-glucopyranoside, and the aglycone luteolin, respectively. Finally, the NMR analysis of compounds 19 and 23 permitted us to attribute these compounds to ferulic acid and caffeoyl-ethyl ester, respectively [31,32] (Figure 5).
2.6. Quantitative Analysis of Caffeoyl Quinic Derivatives (2, 6, 9, 17) in the Eco-Sustainable Extracts of Artichoke Leaves
With the aim of accurately quantifying CQAs in different eco-sustainable extracts, LC-ESI/QTrap/MS/MS analysis was performed. For this purpose, Multiple Reaction Monitoring (MRM), a highly precise tandem mass spectrometry technique, was used to assess the amount (mg/g of extract) of each compound in the extracts based on the selected transitions for the MRM experiments [34,35]. The MRM transitions were chosen on the basis of the fragmentation pattern shown by each metabolite in the ESI/MS/MS spectrum. Therefore, for the [M−H]− pseudomolecular ion at m/z 353 (2, 6), the transition 353→191 corresponding to [quinic acid-H]− was chosen for MRM analysis [36,37]. In turn, the [M−H]− pseudomolecular ion at m/z 515 (9, 17) showed a main product ion at m/z 353 due to [caffeoyl quinic acid-H]−. Therefore, the transition 515→353 was chosen for MRM analysis of compounds 9 and 17 [38]. The most abundant compound in both extracts was 5-caffeoyl quinic acid (6), with values of 9.310 and 7.603 mg/g extract in EtOH:H2O (75:25) and EtOH:H2O (50:50), respectively, followed by 1,5-dicaffeoyl quinic acid (17), which occurred in EtOH:H2O (75:25) and EtOH:H2O (50:50) extracts at concentrations of 2.410 and 2.300 mg/g extract, respectively; compounds 2 and 6 were present in both extracts in very low concentrations (Table 2 and Table S4).
Table 2.
Quantitative results of compounds 2, 6, 9, and 17 (mg/g extract ± SD) in the eco-sustainable extracts of Cynara cardunculus (Carciofo di Paestum PGI) leaves.
| Maceration | 3-Caffeoyl Quinic Acid (2) | 5-Caffeoyl Quinic Acid (6) | 1,3-Dicaffeoyl Quinic Acid (9) | 1,5-Dicaffeoyl Quinic Acid (17) |
|---|---|---|---|---|
| EtOH:H2O (50:50) | 0.069 ± 0.003 | 7.603 ± 0.112 | 0.006 ± 0.001 | 2.300 ± 0.101 |
| EtOH:H2O (75:25) | 0.079 ± 0.007 | 9.310 ± 0.825 | 0.014 ± 0.003 | 2.410 ± 0.159 |
3. Materials and Methods
3.1. Sample Collection and Extraction
Leaves of C. cardunculus subsp. scolymus. cv. “Carciofo di Paestum” PGI were collected at Paestum, Salerno, Italy, in March 2023. Successively, leaves were dried on the bench at room temperature and in an oven at 30 °C. In the second step, to increase the surface of the leaf in contact with the solvent, the dried leaves were reduced into small pieces using a knife. The extracts were prepared using EtOH:H2O (75:25, 50:50) as solvents. In detail, 10 g of dried C. scolymus leaves were extracted with 200 mL of solvent mixture at room temperature (3 days, three times). After filtration, the solvent was evaporated with rotavapor to afford 2.20 g and 3.24 g of EtOH:H2O (75:25) and EtOH:H2O (50:50) of dried extracts, respectively.
3.2. Total Phenolic Content, Total Flavonoid Content, TEAC Assays, FRAP Assays
Folin–Ciocalteu, DPPH, TEAC, and FRAP assays for each extract, repeated in triplicate, were performed as previously reported, with slight modifications [9,39,40,41,42].
3.3. Cell Culture
The Caco-2 cell line was obtained from ECACC (Salisbury, UK) and treated as reported in the Supplementary Materials [43].
3.4. MTT Viability Test
The viability test was estimated using an MTT assay, as previously reported [43], to find any cytotoxic activity of the extract in differentiated Caco-2 cells (Salisbury, UK) (21 days post-seeding) (Supplementary Materials).
3.5. Determination of Intracellular Reactive Oxygen Species (ROS) Production
ROS release in Caco-2 cells was evaluated using the fluorescent probe H2-DCF-DA, as reported in a previous paper by Deiana et al. [44], with minor modifications (Supplementary Materials). For the extracts, a range of 2.5–50 μg/mL was tested.
3.6. Statistical Analyses
Data were analyzed by means of software GraphPad Prism 5 (GraphPad software, San Diego, CA, USA), using one-way analysis of variance (ANOVA) followed by post hoc Tukey’s test. Levels of p < 0.05 were considered statistically significant.
3.7. LC-ESI/HRMSMS Analysis of “Carciofo di Peastum” PGI Leaves Extracts
The extracts of C. cardunculus subsp. scolymus leaves were analyzed, in negative ion mode, through LC-ESI/HRMS using an HPLC with a hybrid mass spectrometer combining the linear trap quadrupole (LTQ) and the Orbitrap mass analyzer (ThermoScientific, San Jose, CA, USA); the same volume of 5 μL (concentration 1 mg/mL) and the same HPLC conditions were used for both samples (Supplementary Materials).
3.8. Isolation of Specialized Metabolites and 1H NMR Data Analysis
The EtOH:H2O (50:50) extract of the leaves was directly submitted to RP-HPLC-UV separation, allowing for the purification of compounds 2, 6, 9, and 17. Moreover, 3 g of the same extract was fractionated with Sephadex LH-20, and 80 fractions were obtained. Different fractions were purified through HPLC-RI, allowing for the purification of compounds 1, 11–14, and 18. Other fractions were purified with RP-HPLC-UV in the same condition applied to the HPLC-UV of the extract, allowing for the purification of compounds 8, 19, and 23. Fraction 78 corresponded to compound 20 (3.1 mg) (Supplementary Materials).
After purification, each compound was diluted in methanol-d4 (Merck, Milan, Italy) using a 5 mm tube, and NMR spectra were performed on a Bruker Ascend-600 spectrometer (Bruker BioSpin GmBH, Rheinstetten, Germany). Monodimensional and bidimensional NMR experiments were acquired (Supplementary Materials).
3.9. Quantitative Analysis of Caffeoyl, Dicaffeoyl, and Quinic Acid Derivatives (CQAs)
Quantitative analyses of CQAs derivatives were achieved, in triplicate, on an LC-ESI/QTrap/MS system (Sciex, Milan, Italy) using MRM mode. Specific chromatographic conditions were applied (Supplementary Materials). Different solutions of ES (0.001, 0.01, 0.1, 2.5, 10.0, 12.0, 15.0, and 17.0 µg/mL) were employed. The chromatographic method was assessed for calibration curve linearity, accuracy, and both intra- and inter-day precision. In detail, the acquisition was achieved three times in the same day (intraday) and in three different days (interday). The limit of quantification (LOD) and the limit of detection (LOD) were calculated [45].
4. Conclusions
In this study, the potential use of two eco-sustainable extracts (EtOH:H2O 50:50 and 75:25) of “Carciofo di Paestum” leaves for the development of food supplements was investigated.
EtOH:H2O 50:50 and 75:25 extracts showed high phenolic concentrations, with values of 167.48 mg GAE/g and 153.41 mg GAE/g, respectively. Flavonoid content followed a similar trend, with 101.02 mg rutin/g and 67.49 mg rutin/g. Moreover, the results showed the interesting antioxidant potential of the extracts across the different methods used for evaluation, with values of 1.90 mM and 1.81 mM for TEAC and an IC50 of 106.31 µg/mL and 128.21 µg/mL for DPPH and for EtOH:H2O 50:50 and 75:25, respectively. Finally, the FRAP assay showed a similar trend with values of 1.68 mM of FeSO4/g for EtOH:H2O 50:50 extract and 1.58 mM of FeSO4/g for EtOH:H2O 75:25 extract. To confirm the radical scavenging activity, an in vitro test on Caco-2 cell cultures was performed, revealing a dose-dependent antioxidant effect of both extracts, starting at 10 μg/mL. Qualitative analysis of both extracts through LC-ESI/HRMSMS was performed to identify the bioactive compounds potentially responsible for the antioxidant activity. In this way, 28 compounds were identified, mainly belonging to sesquiterpenes, quinic acid derivatives, and flavonoids, of which the structures were unambiguously assigned through 1D- and 2D-NMR. Among the identified compounds, our attention was focused on caffeoyl, dicaffeoyl, and quinic acid derivatives (CQAs), key markers of C. scolymus known for their wide-ranging therapeutic properties, including antioxidant, antibacterial, anticancer, hepatoprotective, cardioprotective, anti-inflammatory, antipyretic, neuro-protective, antiviral, antimicrobial, anti-hypertensive, and free radical scavenging effects [15,46,47]. Therefore, quantitative analysis of CQAs was performed using LC-ESI/QTrap/MS/MS. This analysis indicates that both eco-sustainable extracts contain a remarkable level of these bioactive compounds, supporting their potential for development into antioxidant-based food supplements.
Acknowledgments
The authors thank TerraOrti, Eboli (SA), Italy, for providing globe artichokes. Cynara cardunculus subsp. scolymus “Carciofo di Paestum” PGI as a kind gift.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/plants13243591/s1. Plant material; isolation of specialized metabolites, LC-ESI/HRMS/MS analysis; NMR and data processing; total phenolic content, total flavonoid content, DPPH, TEAC, and FRAP assays; Table S1: Phenolic content and antioxidant activity of eco-sustainable extracts of “Carciofo di Paestum” leaves; quantitative analysis of caffeoyl, dicaffeoyl, and quinic acid derivatives (CQAs); Table S2. 1H (600 MHz) and 13C (150 MHz) NMR data of compounds 1, 11, 12, and 18 (CD3OD); Table S3. 1H (600 MHz) and 13C (150 MHz) NMR data of compounds 2, 6, 9, and 17 (CD3OD); Table S4. LC–MS/MS conditions for quantitation of caffeoyl, dicaffeoyl, and quinic acid derivatives (CQAs) in negative ion MRM mode.
Author Contributions
Conceptualization, A.C., M.M. and S.P.; software, A.C., R.C., M.P.M. and G.S.; validation, M.M. and M.D.; formal analysis, A.C., R.C., M.P.M. and G.S.; investigation, A.C., R.C., M.P.M. and G.S.; resources, M.D. and S.P.; data curation, A.C., M.M. and M.D.; writing—original draft preparation, A.C. and M.P.M.; writing—review and editing, M.M., M.D. and S.P.; visualization, M.M.; supervision, S.P.; funding acquisition, S.P. All authors have read and agreed to the published version of the manuscript.
Data Availability Statement
The data presented in this study are available in the Supplementary Materials.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding Statement
This study was carried out within the Agritech National Research Center and received funding from the European Union Next-GenerationEU (PIANO NAZIONALE DI RIPRESA E RESILIENZA (PNRR)-MISSIONE 4 COMPONENTE 2, INVESTIMENTO 1.4-D.D. 1032 17/06/2022, CN00000022). This manuscript reflects only the authors’ views and opinions, and neither the European Union nor the European Commission can be considered responsible for them.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Ayuso P., Quizhpe J., de los Ángeles Rosell M., Peñalver R., Nieto G. Bioactive Compounds, Health Benefits and Food Applications of Artichoke (Cynara scolymus L.) and Artichoke By-Products: A Review. Appl. Sci. 2024;14:4940. doi: 10.3390/app14114940. [DOI] [Google Scholar]
- 2.Pedrali D., Zuccolo M., Giupponi L., Sala S., Giorgi A. Characterization and Future Distribution Prospects of “Carciofo di Malegno” Landrace for Its In Situ Conservation. Plants. 2024;13:680. doi: 10.3390/plants13050680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lattanzio V., Kroon P.A., Linsalata V., Cardinali A. Globe artichoke: A functional food and source of nutraceutical ingredients. J. Funct. Foods. 2009;1:131–144. doi: 10.1016/j.jff.2009.01.002. [DOI] [Google Scholar]
- 4.Borsini A.A., Llavata B., Umaña M., Cárcel J.A. Artichoke by Products as a Source of Antioxidant and Fiber: How It Can Be Affected by Drying Temperature. Foods. 2021;10:459. doi: 10.3390/foods10020459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Laghezza Masci V., Alicandri E., Antonelli C., Paolacci A.R., Marabottini R., Tomassi W., Scarascia Mugnozza G., Tiezzi A., Garzoli S., Vinciguerra V., et al. Cynara cardunculus L. var. scolymus L. Landrace “Carciofo Ortano” as a Source of Bioactive Compounds. Plants. 2024;13:761. doi: 10.3390/plants13060761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morone P., Koutinas A., Gathergood N., Arshadi M., Matharu A. Food waste: Challenges and opportunities for enhancing the emerging bio-economy. J. Clean. Prod. 2019;221:10–16. doi: 10.1016/j.jclepro.2019.02.258. [DOI] [Google Scholar]
- 7.Food and Agriculture Organization of the United Nations FAOSTAT. Crops and Livestock Products. 2022. [(accessed on 8 March 2024)]. Available online: https://www.fao.org/faostat/en/#data/QCL.
- 8.Rana R.L., Bux C., Lombardi M.J.B.F.J. Trends in scientific literature on the environmental sustainability of the artichoke (Cynara cardunculus L. spp.) supply chain. Br. Food J. 2022;125:2315–2332. doi: 10.1108/BFJ-07-2022-0571. [DOI] [Google Scholar]
- 9.Cerulli A., Masullo M., Pizza C., Piacente S. Metabolite Profiling of “Green” Extracts of Cynara cardunculus subsp. scolymus, Cultivar “Carciofo di Paestum” PGI by 1H NMR and HRMS-Based Metabolomics. Molecules. 2022;27:3328. doi: 10.3390/molecules27103328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bonasia A., Conversa G., Lazzizera C., Elia A. Characterization of Targeted Phenolic Compounds in Globe Artichoke Heads and Waste from Vegetatively and “Seed”-Propagated Genotypes. Plants. 2023;12:2579. doi: 10.3390/plants12132579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Montesano V., Negro D., Sonnante G., Laghetti G., Urbano M. Polyphenolic Compound Variation in Globe Artichoke Cultivars as Affected by Fertilization and Biostimulants Application. Plants. 2022;11:2067. doi: 10.3390/plants11152067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Colombo R., Moretto G., Pellicorio V., Papetti A. Globe Artichoke (Cynara scolymus L.) By-Products in Food Applications: Functional and Biological Properties. Foods. 2024;13:1427. doi: 10.3390/foods13101427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu W., Li J., Zhang X., Zu Y., Yang Y., Liu W., Xu Z., Gao H., Sun X., Jiang X., et al. Current Advances in Naturally Occurring Caffeoylquinic Acids: Structure, Bioactivity, and Synthesis. J. Agric. Food Chem. 2020;68:10489–10516. doi: 10.1021/acs.jafc.0c03804. [DOI] [PubMed] [Google Scholar]
- 14.Clifford M.N., Jaganath I.B., Ludwig I.A., Crozier A. Chlorogenic acids and the acyl-quinic acids: Discovery, biosynthesis, bioavailability and bioactivity. Nat. Prod. Rep. 2017;34:1391–1421. doi: 10.1039/C7NP00030H. [DOI] [PubMed] [Google Scholar]
- 15.Li L., Su C., Chen X., Wang Q., Jiao W., Luo H., Tang J., Wang W., Li S., Guo S. Chlorogenic Acids in Cardiovascular Disease: A Review of Dietary Consumption, Pharmacology, and Pharmacokinetics. J. Agric. Food Chem. 2020;68:6464–6484. doi: 10.1021/acs.jafc.0c01554. [DOI] [PubMed] [Google Scholar]
- 16.Capello C., Fischer U., Hungerbühler K. What is a green solvent? A comprehensive framework for the environmental assessment of solvents. Green Chem. 2007;9:927–934. doi: 10.1039/b617536h. [DOI] [Google Scholar]
- 17.Martins R., Barbosa A., Advinha B., Sales H., Pontes R., Nunes J. Green Extraction Techniques of Bioactive Compounds: A State-of-the-Art Review. Processes. 2023;11:2255. doi: 10.3390/pr11082255. [DOI] [Google Scholar]
- 18.Matić M., Stupar A., Pezo L., Đerić Ilić N., Mišan A., Teslić N., Pojić M., Mandić A. Eco-Friendly Extraction: A green approach to maximizing bioactive extraction from pumpkin (Curcubita moschata L.) Food Chem. X. 2024;22:101290. doi: 10.1016/j.fochx.2024.101290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nguyen V., Taine E.G., Meng D., Cui T., Tan W. Chlorogenic Acid: A Systematic Review on the Biological Functions, Mechanistic Actions, and Therapeutic Potentials. Nutrients. 2024;16:924. doi: 10.3390/nu16070924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cerulli A., Napolitano A., Masullo M., Hošek J., Pizza C., Piacente S. Chestnut shells (Italian cultivar “Marrone di Roccadaspide” PGI): Antioxidant activity and chemical investigation with in depth LC-HRMS/MSn rationalization of tannins. Food Res Int. 2020;129:108787. doi: 10.1016/j.foodres.2019.108787. [DOI] [PubMed] [Google Scholar]
- 21.Marakhova A., Zhilkina V.Y., Elapov A., Sachivkina N., Samorodov A., Pupykina K., Krylova I., Kezimana P., Stoynova A.M., Venkatesan R., et al. The Development of a Method for Obtaining Tripleurospermum inodorum (L.) Sch. Bip. Herb Extract Enriched with Flavonoids and an Evaluation of Its Biological Activity. Plants. 2024;13:1629. doi: 10.3390/plants13121629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jiménez-Moreno N., Cimminelli M.J., Volpe F., Ansó R., Esparza I., Mármol I., Rodríguez-Yoldi M.J., Ancín-Azpilicueta C. Phenolic Composition of Artichoke Waste and Its Antioxidant Capacity on Differentiated Caco-2 Cells. Nutrients. 2019;11:1723. doi: 10.3390/nu11081723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vacca M., Pinto D., Annunziato A., Ressa A., Calasso M., Pontonio E., Celano G., De Angelis M. Gluten-Free Bread Enriched with Artichoke Leaf Extract In Vitro Exerted Antioxidant and Anti-Inflammatory Properties. Antioxidants. 2023;12:845. doi: 10.3390/antiox12040845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Corbetta P., Lonati E., Pagliari S., Mauri M., Cazzaniga E., Botto L., Campone L., Palestini P., Bulbarelli A. Flavonoids-Enriched Vegetal Extract Prevents the Activation of NFκB Downstream Mechanisms in a Bowel Disease In Vitro Model. Int. J. Mol. Sci. 2024;25:7869. doi: 10.3390/ijms25147869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cerulli A., Lauro G., Masullo M., Cantone V., Olas B., Kontek B., Nazzaro F., Bifulco G., Piacente S. Cyclic Diarylheptanoids from Corylus avellana Green Leafy Covers: Determination of Their Absolute Configurations and Evaluation of Their Antioxidant and Antimicrobial Activities. J. Nat. Prod. 2017;80:1703–1713. doi: 10.1021/acs.jnatprod.6b00703. [DOI] [PubMed] [Google Scholar]
- 26.Mahmood N., Piacente S., Burke A., Khan A., Pizza C. Constituents of Cuscuto Reflexa are anti-HIV Agents. Antivir. Chem. Chemother. 1997;8:70–74. doi: 10.1177/095632029700800108. [DOI] [Google Scholar]
- 27.Hosseini S.H., Masullo M., Cerulli A., Martucciello S., Ayyari M., Pizza C., Piacente S. Antiproliferative Cardenolides from the Aerial Parts of Pergularia tomentosa. J. Nat. Prod. 2019;82:74–79. doi: 10.1021/acs.jnatprod.8b00630. [DOI] [PubMed] [Google Scholar]
- 28.Farag M.A., El-Ahmady S.H., Elian F.S., Wessjohann L.A. Metabolomics driven analysis of artichoke leaf and its commercial products via UHPLC–q-TOF-MS and chemometrics. Phytochemistry. 2013;95:177–187. doi: 10.1016/j.phytochem.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 29.Liu R., Hsieh K.-L., Liu J.-K. A New Sesquiterpene Lactone from the Leaves of Cynara scolymus (Compositae) Acta Bot. Yunnanica. 2010;31:383–385. doi: 10.3724/SP.J.1143.2009.09062. [DOI] [Google Scholar]
- 30.Shimoda H., Ninomiya K., Nishida N., Yoshino T., Morikawa T., Matsuda H., Yoshikawa M. Anti-Hyperlipidemic sesquiterpenes and new sesquiterpene glycosides from the leaves of artichoke (Cynara scolymus L.): Structure requirement and mode of action. Bioorganic Med. Chem. Lett. 2003;13:223–228. doi: 10.1016/S0960-894X(02)00889-2. [DOI] [PubMed] [Google Scholar]
- 31.Horman I., Badoud R., Ammann W. Food-related applications of one- and two-dimensional high-resolution proton-NMR: Structure and conformation of cynarin. J. Agric. Food Chem. 1984;32:538–540. doi: 10.1021/jf00123a030. [DOI] [Google Scholar]
- 32.Maruta Y., Kawabata J., Niki R. Antioxidative caffeoylquinic acid derivatives in the roots of burdock (Arctium lappa L.) J. Agric. Food Chem. 1995;43:2592–2595. doi: 10.1021/jf00058a007. [DOI] [Google Scholar]
- 33.Wan C., Li S., Liu L., Chen C., Fan S. Caffeoylquinic Acids from the Aerial Parts of Chrysanthemum coronarium L. Plants. 2017;6:10. doi: 10.3390/plants6010010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rhee J.-H., Choi S., Lee J.-E., Hur O.-S., Ro N.-Y., Hwang A.-J., Ko H.-C., Chung Y.-J., Noh J.-J., Assefa A.D. Glucosinolate Content in Brassica Genetic Resources and Their Distribution Pattern within and between Inner, Middle, and Outer Leaves. Plants. 2020;9:1421. doi: 10.3390/plants9111421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maldini M., Montoro P., Pizza C. Phenolic compounds from Byrsonima crassifolia L. bark: Phytochemical investigation and quantitative analysis by LC-ESI MS/MS. J. Pharm. Biomed. Anal. 2011;56:1–6. doi: 10.1016/j.jpba.2011.03.032. [DOI] [PubMed] [Google Scholar]
- 36.Bai C., Zhou X., Yu L., Wu A., Yang L., Chen J., Tang X., Zou W., Wu J., Zhu L. A Rapid and Sensitive UHPLC–MS/MS Method for Determination of Chlorogenic Acid and Its Application to Distribution and Neuroprotection in Rat Brain. Pharmaceuticals. 2023;16:178. doi: 10.3390/ph16020178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang J., Chen M., Ju W., Liu S., Xu M., Chu J., Wu T. Liquid chromatograph/tandem mass spectrometry assay for the simultaneous determination of chlorogenic acid and cinnamic acid in plasma and its application to a pharmacokinetic study. J. Pharm. Biomed. Anal. 2010;51:685–690. doi: 10.1016/j.jpba.2009.09.039. [DOI] [PubMed] [Google Scholar]
- 38.Moglia A., Lanteri S., Comino C., Acquadro A., de Vos R., Beekwilder J. Stress-Induced Biosynthesis of Dicaffeoylquinic Acids in Globe Artichoke. J. Agric. Food Chem. 2008;56:8641–8649. doi: 10.1021/jf801653w. [DOI] [PubMed] [Google Scholar]
- 39.Biel W., Witkowicz R., Piątkowska E., Podsiadło C. Proximate Composition, Minerals and Antioxidant Activity of Artichoke Leaf Extracts. Biol. Trace Elem. Res. 2020;194:589–595. doi: 10.1007/s12011-019-01806-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang M., Xu Y., Xiang J., Zheng B., Yuan Y., Luo D., Fan J. Comparative evaluation on phenolic profiles, antioxidant properties and α-glucosidase inhibitory effects of different milling fractions of foxtail millet. J. Cereal Sci. 2021;99:103217. doi: 10.1016/j.jcs.2021.103217. [DOI] [Google Scholar]
- 41.Shahidi F., Alasalvar C., Liyana-Pathirana C.M. Antioxidant Phytochemicals in Hazelnut Kernel (Corylus avellana L.) and Hazelnut Byproducts. J. Agric. Food Chem. 2007;55:1212–1220. doi: 10.1021/jf062472o. [DOI] [PubMed] [Google Scholar]
- 42.Abu-Reidah I.M., Arráez-Román D., Segura-Carretero A., Fernández-Gutiérrez A. Extensive characterisation of bioactive phenolic constituents from globe artichoke (Cynara scolymus L.) by HPLC–DAD-ESI-QTOF-MS. Food Chem. 2013;141:2269–2277. doi: 10.1016/j.foodchem.2013.04.066. [DOI] [PubMed] [Google Scholar]
- 43.Serreli G., Naitza M.R., Zodio S., Leoni V.P., Spada M., Melis M.P., Boronat A., Deiana M. Ferulic Acid Metabolites Attenuate LPS-Induced Inflammatory Response in Enterocyte-like Cells. Nutrients. 2021;13:3152. doi: 10.3390/nu13093152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Deiana M., Montoro P., Jerković I., Atzeri A., Marijanović Z., Serreli G., Piacente S., Tuberoso C.I.G. First characterization of Pompia intrea candied fruit: The headspace chemical profile, polar extract composition and its biological activities. Food Res. Int. 2019;120:620–630. doi: 10.1016/j.foodres.2018.11.016. [DOI] [PubMed] [Google Scholar]
- 45.Maldini M., Chessa M., Petretto G.L., Montoro P., Rourke J.P., Foddai M., Nicoletti M., Pintore G. Profiling and Simultaneous Quantitative Determination of Anthocyanins in Wild Myrtus communis L. Berries from Different Geographical Areas in Sardinia and their Comparative Evaluation. Phytochem. Anal. 2016;27:249–256. doi: 10.1002/pca.2623. [DOI] [PubMed] [Google Scholar]
- 46.Xue H., Wei M., Ji L. Chlorogenic acids: A pharmacological systematic review on their hepatoprotective effects. Phytomedicine. 2023;118:154961. doi: 10.1016/j.phymed.2023.154961. [DOI] [PubMed] [Google Scholar]
- 47.Lemos M.F., de Andrade Salustriano N., de Souza Costa M.M., Lirio K., da Fonseca A.F.A., Pacheco H.P., Endringer D.C., Fronza M., Scherer R. Chlorogenic acid and caffeine contents and anti-inflammatory and antioxidant activities of green beans of conilon and arabica coffees harvested with different degrees of maturation. J. Saudi Chem. Soc. 2022;26:101467. doi: 10.1016/j.jscs.2022.101467. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data presented in this study are available in the Supplementary Materials.