Abstract
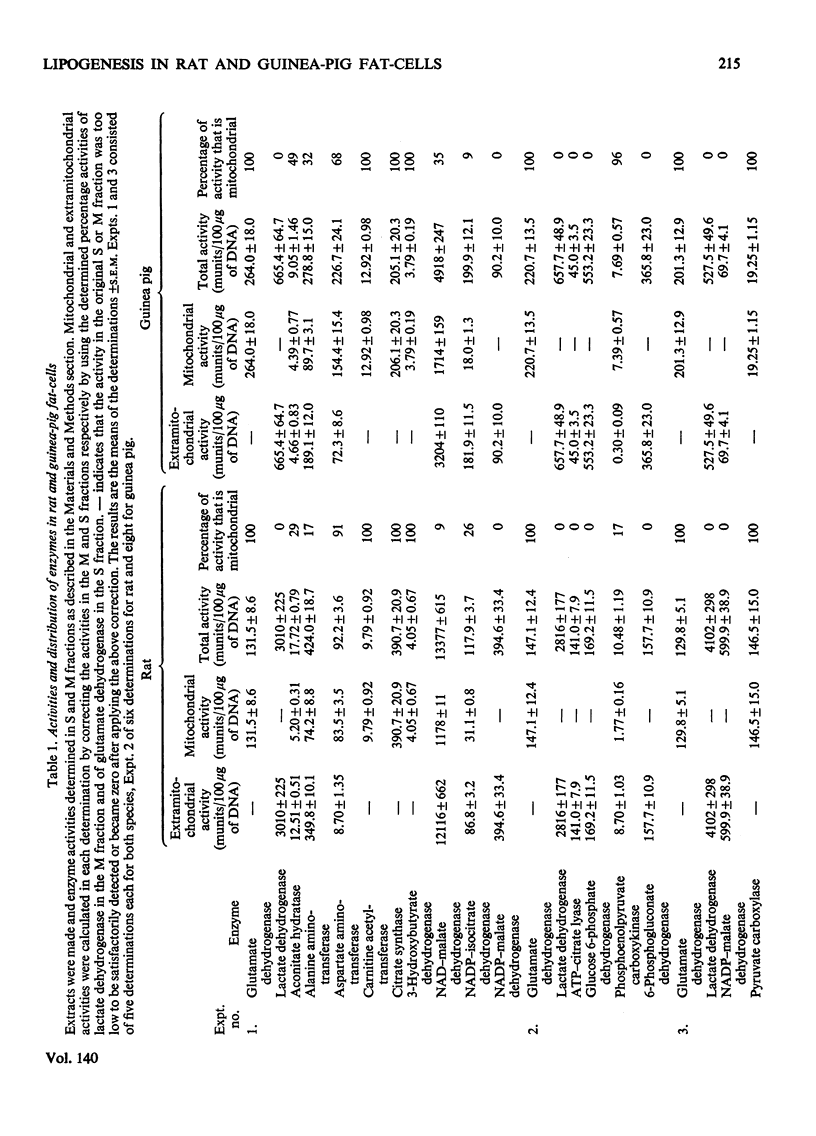
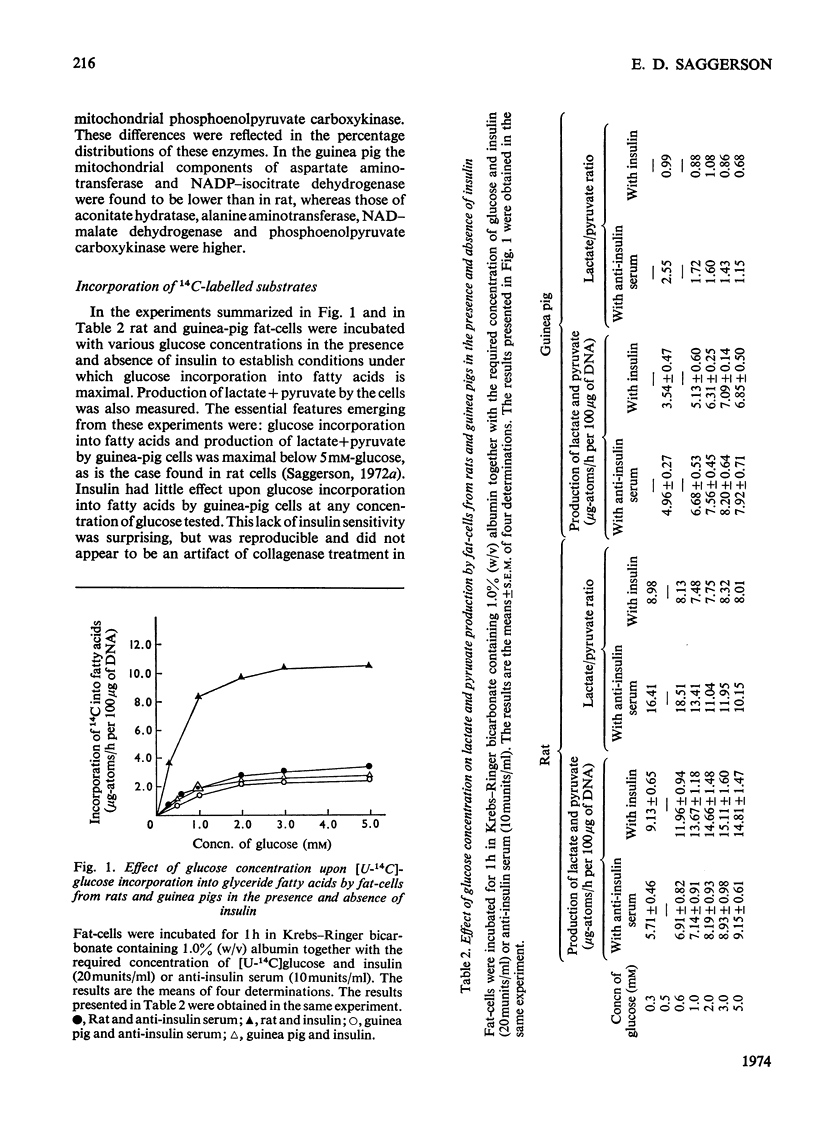
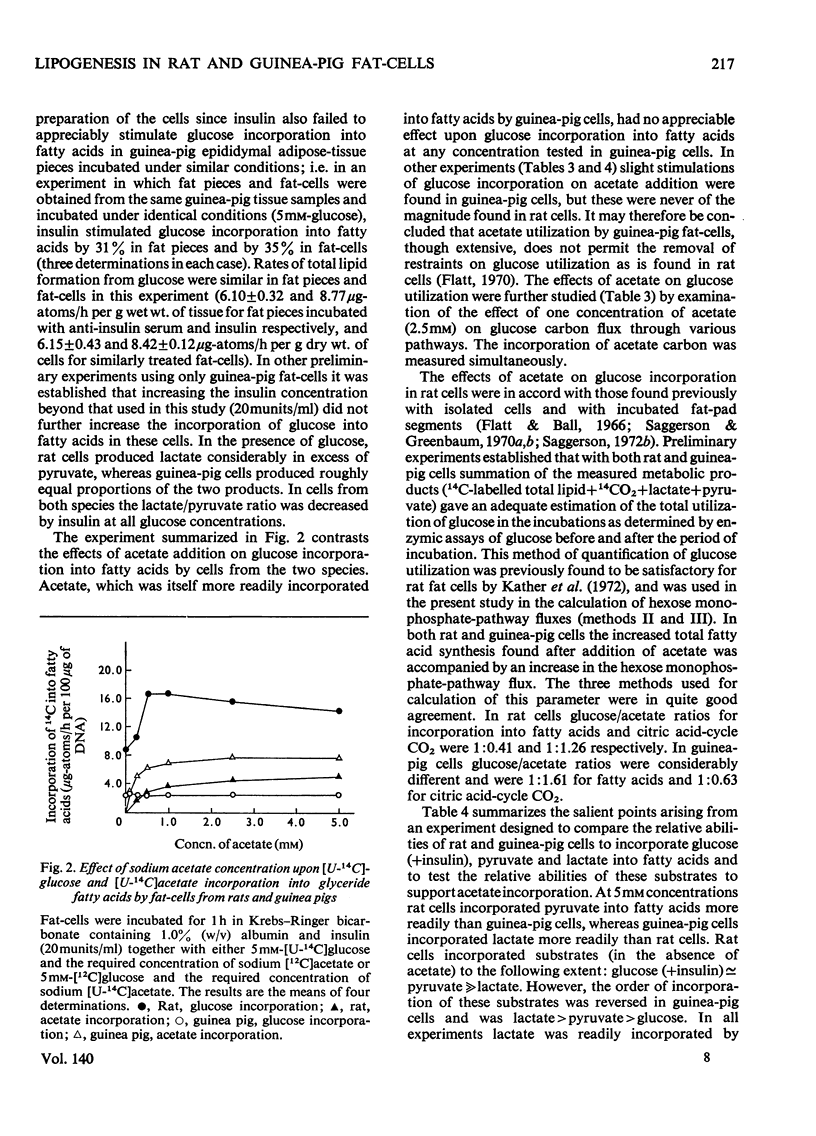
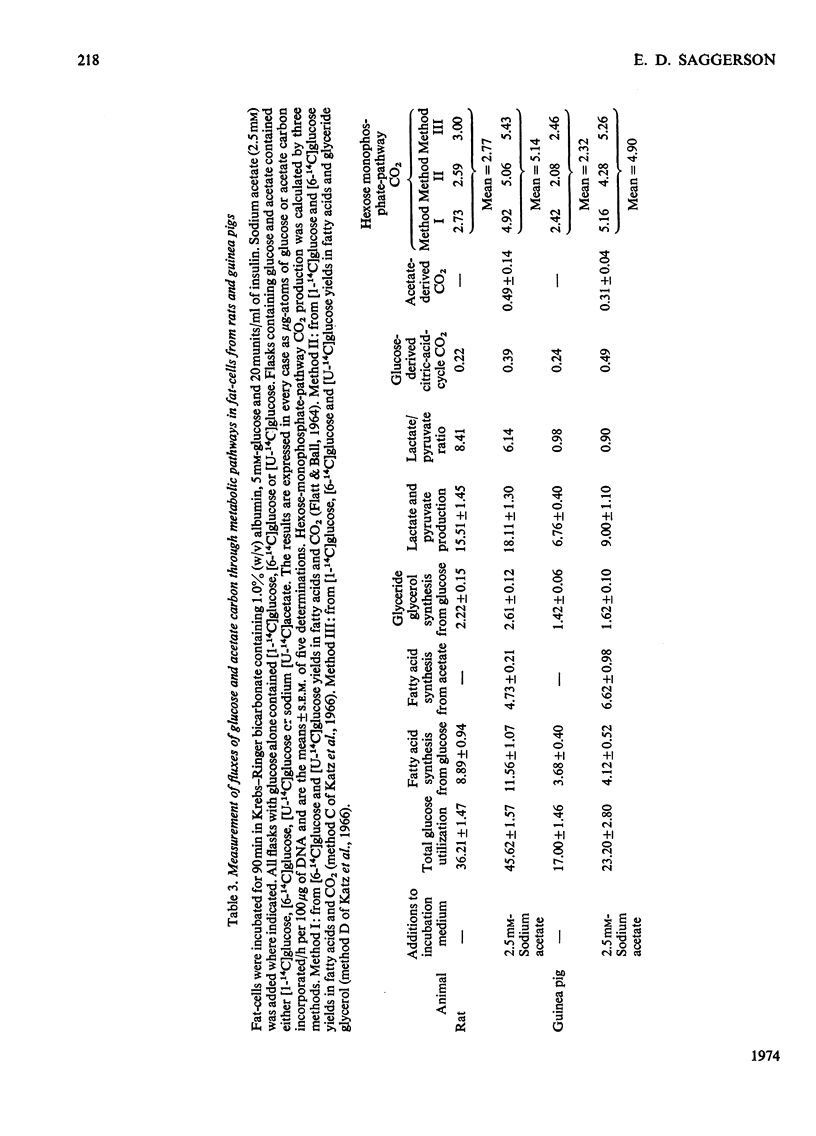
Fat-cells were prepared from rat and guinea-pig epididymal adipose tissue and compared on the basis of the intracellular distributions and activities of enzymes and with respect to their utilization of various U-14C-labelled substrates for lipogenesis. 1. Compared with the rat, guinea-pig extramitochondrial enzyme activities differed in that aconitate hydratase, alanine aminotransferase, ATP–citrate lyase, lactate dehydrogenase, NAD–malate dehydrogenase, NADP–malate dehydrogenase and phosphoenolpyruvate carboxykinase activities were appreciably lower, whereas aspartate aminotransferase, glucose 6-phosphate dehydrogenase, NADP–isocitrate dehydrogenase and 6-phosphogluconate dehydrogenase activities were appreciably higher. Mitochondrial activities of citrate synthase, NADP–isocitrate dehydrogenase and pyruvate carboxylase were appreciably lower, whereas mitochondrial activities of aspartate aminotransferase, glutamate dehydrogenase, NAD–malate dehydrogenase and phosphoenolpyruvate carboxykinase were higher in the guinea pig compared with the rat. 2. In general guinea-pig fat-cells incorporated acetate and lactate into fatty acids more readily than rat fat-cells, whereas rat fat-cells incorporated glucose and pyruvate more readily than guinea-pig fat-cells. 3. Acetate stimulated the incorporation of glucose into fatty acids in rat fat-cells, but had no appreciable effect upon this process in guinea-pig fat-cells. Acetate greatly decreased the incorporation of lactate into fatty acids in cells from both species. 4. Lactate/pyruvate ratios produced by incubation of guinea-pig cells with glucose+insulin were very low compared with those found with rat cells under the same conditions. 5. With glucose (+insulin) or with glucose+acetate (+insulin) as substrates guinea-pig cells produced enough NADPH by the hexose monophosphate pathway to satisfy the NADPH requirements of lipogenesis. In rat fat-cells under the same conditions, hexose monophosphate-pathway NADPH provision was not sufficient to meet the requirements of lipogenesis. 6. These results are discussed, particularly in relationship to the disposition of cytosolic reducing equivalents in the cells.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson D. B., Kauffman R. G. Cellular and enzymatic changes in porcine adipose tissue during growth. J Lipid Res. 1973 Mar;14(2):160–168. [PubMed] [Google Scholar]
- Anson R. W., Ballard F. J. The metabolic fate of the products of citrate cleavage. Adenosine triphosphate-citrate lyase and nicotinamide-adenine dinucleotide phosphate-linked malate dehydrogenase in foetal and adult liver from ruminants and non-ruminants. Biochem J. 1968 Aug;108(5):705–713. doi: 10.1042/bj1080705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arinze I. J., Garber A. J., Hanson R. W. The regulation of gluconeogenesis in mammalian liver. The role of mitochondrial phosphoenolpyruvate carboxykinase. J Biol Chem. 1973 Apr 10;248(7):2266–2274. [PubMed] [Google Scholar]
- BRUCE H. M., PARKES A. S. Feeding and breeding of laboratory animals; a complete cubed diet for mice and rats. J Hyg (Lond) 1949 Jun;47(2):202–208. doi: 10.1017/s0022172400014479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballard F. J., Filsell O. H., Jarrett I. G. Effects of carbohydrate availability on lipogenesis in sheep. Biochem J. 1972 Jan;126(1):193–200. doi: 10.1042/bj1260193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauman D. E., Brown R. E., Davis C. L. Pathways of fatty acid synthesis and reducing equivalent generation in mammary gland of rat, sow, and cow. Arch Biochem Biophys. 1970 Sep;140(1):237–244. doi: 10.1016/0003-9861(70)90028-7. [DOI] [PubMed] [Google Scholar]
- Bauman D. E., DeKay D. E., Ingle D. L., Brown R. E. Effect of glycerol and glucose additions on lipogenesis from acetate in rat and cow mammary tissue. Comp Biochem Physiol B. 1972 Nov 15;43(3):479–486. doi: 10.1016/0305-0491(72)90131-9. [DOI] [PubMed] [Google Scholar]
- Chang H. C., Lane M. D. The enzymatic carboxylation of phosphoenolpyruvate. II. Purification and properties of liver mitochondrial phosphoenolpyruvate carboxykinase. J Biol Chem. 1966 May 25;241(10):2413–2420. [PubMed] [Google Scholar]
- Del Boca J., Flatt J. P. Fatty acid synthesis from glucose and acetate and the control of lipogenesis in adipose tissue. Eur J Biochem. 1969 Nov;11(1):127–134. doi: 10.1111/j.1432-1033.1969.tb00749.x. [DOI] [PubMed] [Google Scholar]
- Denton R. M., Yorke R. E., Randle P. J. Measurement of concentrations of metabolites in adipose tissue and effects of insulin, alloxan-diabetes and adrenaline. Biochem J. 1966 Aug;100(2):407–419. doi: 10.1042/bj1000407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FLATT J. P., BALL E. G. STUDIES ON THE METABOLISM OF ADIPOSE TISSUE. XV. AN EVALUATION OF THE MAJOR PATHWAYS OF GLUCOSE CATABOLISM AS INFLUENCED BY INSULIN AND EPINEPHRINE. J Biol Chem. 1964 Mar;239:675–685. [PubMed] [Google Scholar]
- Flatt J. P., Ball E. G. Studies on the metabolism of adipose tissue. XIX. An evaluation of the major pathways of glucose catabolism as influenced by acetate in the presence of insulin. J Biol Chem. 1966 Jun 25;241(12):2862–2869. [PubMed] [Google Scholar]
- Flatt J. P. Conversion of carbohydrate to fat in adipose tissue: an energy-yielding and, therefore, self-limiting process. J Lipid Res. 1970 Mar;11(2):131–143. [PubMed] [Google Scholar]
- Garber A. J., Hanson R. W. The interrelationships of the various pathways forming gluconeogenic precursors in guinea pig liver mitochondria. J Biol Chem. 1971 Feb 10;246(3):589–598. [PubMed] [Google Scholar]
- Greenbaum A. L., Gumaa K. A., McLean P. The distribution of hepatic metabolites and the control of the pathways of carbohydrate metabolism in animals of different dietary and hormonal status. Arch Biochem Biophys. 1971 Apr;143(2):617–663. doi: 10.1016/0003-9861(71)90247-5. [DOI] [PubMed] [Google Scholar]
- Gul B., Dils R. Enzymic changes in rabbit and rat mammary gland during the lactation cycle. Biochem J. 1969 Apr;112(3):293–301. doi: 10.1042/bj1120293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gumaa K. A., Greenbaum A. L., McLean P. Adaptive changes in satellite systems related to lipogenesis in rat and sheep mammary gland and in adipose tissue. Eur J Biochem. 1973 Apr 2;34(1):188–198. doi: 10.1111/j.1432-1033.1973.tb02745.x. [DOI] [PubMed] [Google Scholar]
- Halperin M. L. Studies on the conversion of pyruvate into fatty acids in white adipose tissue. Effects of insulin, alloxan-diabetes and starvation. Biochem J. 1971 Sep;124(3):615–621. doi: 10.1042/bj1240615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson R. W., Ballard F. J. The relative significance of acetate and glucose as precursors for lipid synthesis in liver and adipose tissue from ruminants. Biochem J. 1967 Nov;105(2):529–536. doi: 10.1042/bj1050529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardwick D. C. The fate of acetyl groups derived from glucose in the isolated perfused goat udder. Biochem J. 1966 Apr;99(1):228–231. doi: 10.1042/bj0990228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KORNACKER M. S., LOWENSTEIN J. M. CITRATE AND THE CONVERSION OF CARBOHYDRATE INTO FAT. THE ACTIVITIES OF CITRATE-CLEAVAGE ENZYME AND ACETATE THIOKINASE IN LIVERS OF STARVED AND RE-FED RATS. Biochem J. 1965 Jan;94:209–215. doi: 10.1042/bj0940209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kather H., Rivera M., Brand K. Interrelationship and control of glucose metabolism and lipogenesis in isolated fat-cells. Effect of the amount of glucose uptake on the rates of the pentose phosphate cycle and of fatty acid synthesis. Biochem J. 1972 Aug;128(5):1089–1096. doi: 10.1042/bj1281089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz J., Landau B. R., Bartsch G. E. The pentose cycle, triose phosphate isomerization, and lipogenesis in rat adipose tissue. J Biol Chem. 1966 Feb 10;241(3):727–740. [PubMed] [Google Scholar]
- Katz J., Wals P. A. Effect of phenazine methosulfate on lipogenesis. J Biol Chem. 1970 May 25;245(10):2546–2548. [PubMed] [Google Scholar]
- Katz J., Wals P. A. Pentose cycle and reducing equivalents in rat mammary-gland slices. Biochem J. 1972 Jul;128(4):879–899. doi: 10.1042/bj1280879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kneer P., Ball E. G. Studies on the metabolism of adipose tissue. XXI. An evaluation of the major pathways of pyruvate metabolism. J Biol Chem. 1968 Jun 10;243(11):2863–2870. [PubMed] [Google Scholar]
- LEHNINGER A. L., SUDDUTH H. C., WISE J. B. D-beta-Hydroxybutyric dehydrogenase of muitochondria. J Biol Chem. 1960 Aug;235:2450–2455. [PubMed] [Google Scholar]
- Lardy H. A. Gluconeogenesis: pathways and hormonal regulation. Harvey Lect. 1966;60:261–278. [PubMed] [Google Scholar]
- Lardy H. A., Paetkau V., Walter P. Paths of carbon in gluconeogenesis and lipogenesis: the role of mitochondria in supplying precursors of phosphoenolpyruvate. Proc Natl Acad Sci U S A. 1965 Jun;53(6):1410–1415. doi: 10.1073/pnas.53.6.1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin B. R., Denton R. M. Metabolism of pyruvate and malate by isolated fat-cell mitochondria. Biochem J. 1971 Nov;125(1):105–113. doi: 10.1042/bj1250105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin B. R., Denton R. M. The intracellular localization of enzymes in white-adipose-tissue fat-cells and permeability properties of fat-cell mitochondria. Transfer of acetyl units and reducing power between mitochondria and cytoplasm. Biochem J. 1970 May;117(5):861–877. doi: 10.1042/bj1170861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Hea E. K., Leveille G. A. Lipid metabolism in isolated adipose tissue of the domestic pig (Sus domesticus). Comp Biochem Physiol. 1968 Sep;26(3):1081–1089. doi: 10.1016/0010-406x(68)90028-5. [DOI] [PubMed] [Google Scholar]
- O'Hea E. K., Leveille G. A. Significance of adipose tissue and liver as sites of fatty acid synthesis in the pig and the efficiency of utilization of various substrates for lipogenesis. J Nutr. 1969 Nov;99(3):338–344. doi: 10.1093/jn/99.3.338. [DOI] [PubMed] [Google Scholar]
- RODBELL M. METABOLISM OF ISOLATED FAT CELLS. I. EFFECTS OF HORMONES ON GLUCOSE METABOLISM AND LIPOLYSIS. J Biol Chem. 1964 Feb;239:375–380. [PubMed] [Google Scholar]
- Rognstad R., Katz J. The balance of pyridine nucleotides and ATP in adipose tissue. Proc Natl Acad Sci U S A. 1966 May;55(5):1148–1156. doi: 10.1073/pnas.55.5.1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rognstad R., Katz J. The effect of 2,4-dinitrophenol on adipose-tissue metabolism. Biochem J. 1969 Feb;111(4):431–444. doi: 10.1042/bj1110431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saggerson E. D., Greenbaum A. L. The effect of dietary and hormonal conditions on the activities of glycolytic enzymes in rat epididymal adipose tissue. Biochem J. 1969 Nov;115(3):405–417. doi: 10.1042/bj1150405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saggerson E. D., Greenbaum A. L. The regulation of triglyceride synthesis and fatty acid synthesis in rat epididymal adipose tissue. Effects of altered dietary and hormonal conditions. Biochem J. 1970 Sep;119(2):221–242. doi: 10.1042/bj1190221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saggerson E. D., Greenbaum A. L. The regulation of triglyceride synthesis and fatty acid synthesis in rat epididymal adipose tissue. Biochem J. 1970 Sep;119(2):193–219. doi: 10.1042/bj1190193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saggerson E. D. The regulation of glyceride synthesis in isolated white-fat cells. The effects of acetate, pyruvate, lactate, palmitate, electron-acceptors, uncoupling agents and oligomycin. Biochem J. 1972 Aug;128(5):1069–1078. doi: 10.1042/bj1281069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saggerson E. D. The regulation of glyceride synthesis in isolated white-fat cells. The effects of palmitate and lipolytic agents. Biochem J. 1972 Aug;128(5):1057–1067. doi: 10.1042/bj1281057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saggerson E. D., Tomassi G. The regulation of glyceride synthesis from pyruvate in isolated fat cells. The effects of palmitate and alteration of dietary status. Eur J Biochem. 1971 Nov 11;23(1):109–117. doi: 10.1111/j.1432-1033.1971.tb01597.x. [DOI] [PubMed] [Google Scholar]
- Strong C. R., Dils R. Fatty acids synthesized by mammary gland slices from lactating guinea pig and rabbit. Comp Biochem Physiol B. 1972 Nov 15;43(3):643–652. doi: 10.1016/0305-0491(72)90149-6. [DOI] [PubMed] [Google Scholar]
- UTTER M. F., KEECH D. B. PYRUVATE CARBOXYLASE. I. NATURE OF THE REACTION. J Biol Chem. 1963 Aug;238:2603–2608. [PubMed] [Google Scholar]
- Williamson D. H., Lund P., Krebs H. A. The redox state of free nicotinamide-adenine dinucleotide in the cytoplasm and mitochondria of rat liver. Biochem J. 1967 May;103(2):514–527. doi: 10.1042/bj1030514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson J. R., Browning E. T., Scholz R. Control mechanisms of gluconeogenesis and ketogenesis. I. Effects of oleate on gluconeogenesis in perfused rat liver. J Biol Chem. 1969 Sep 10;244(17):4607–4616. [PubMed] [Google Scholar]
- Wimhurst J. M., Manchester K. L. Some aspects of the kinetics of rat liver pyruvate carboxylase. Biochem J. 1970 Nov;120(1):79–93. doi: 10.1042/bj1200079. [DOI] [PMC free article] [PubMed] [Google Scholar]


