Abstract
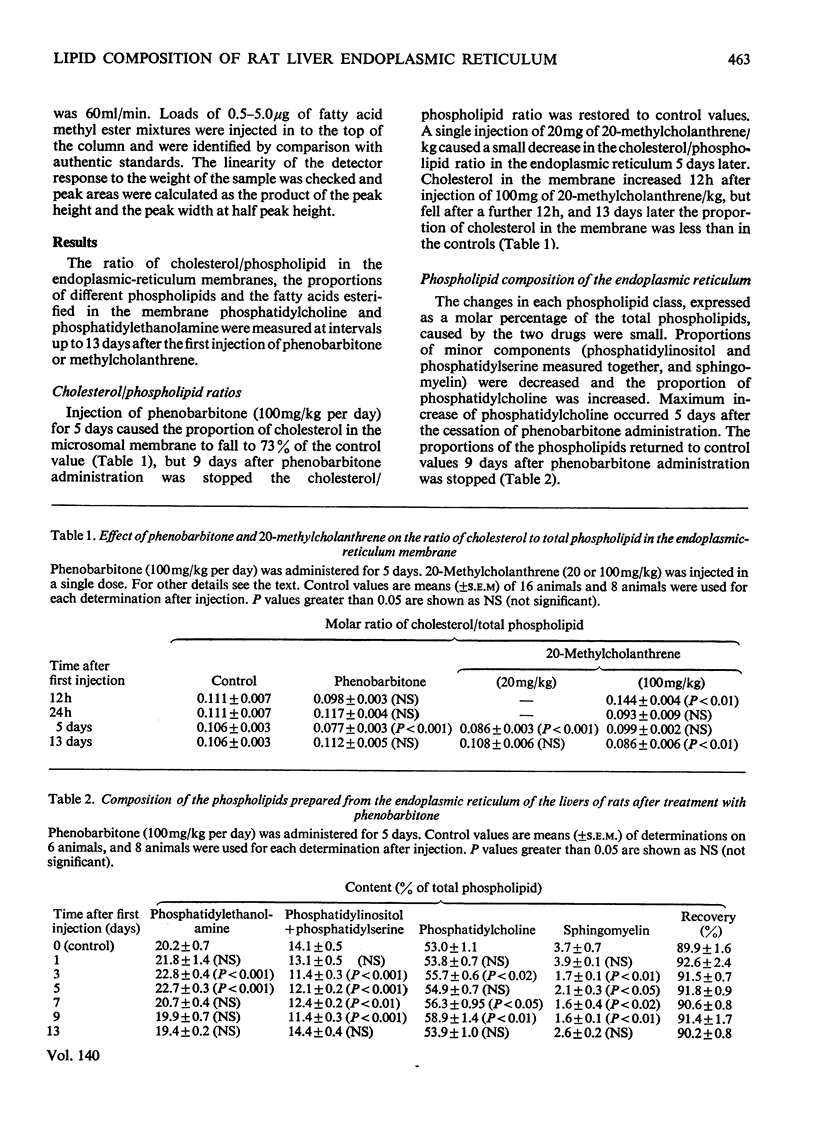
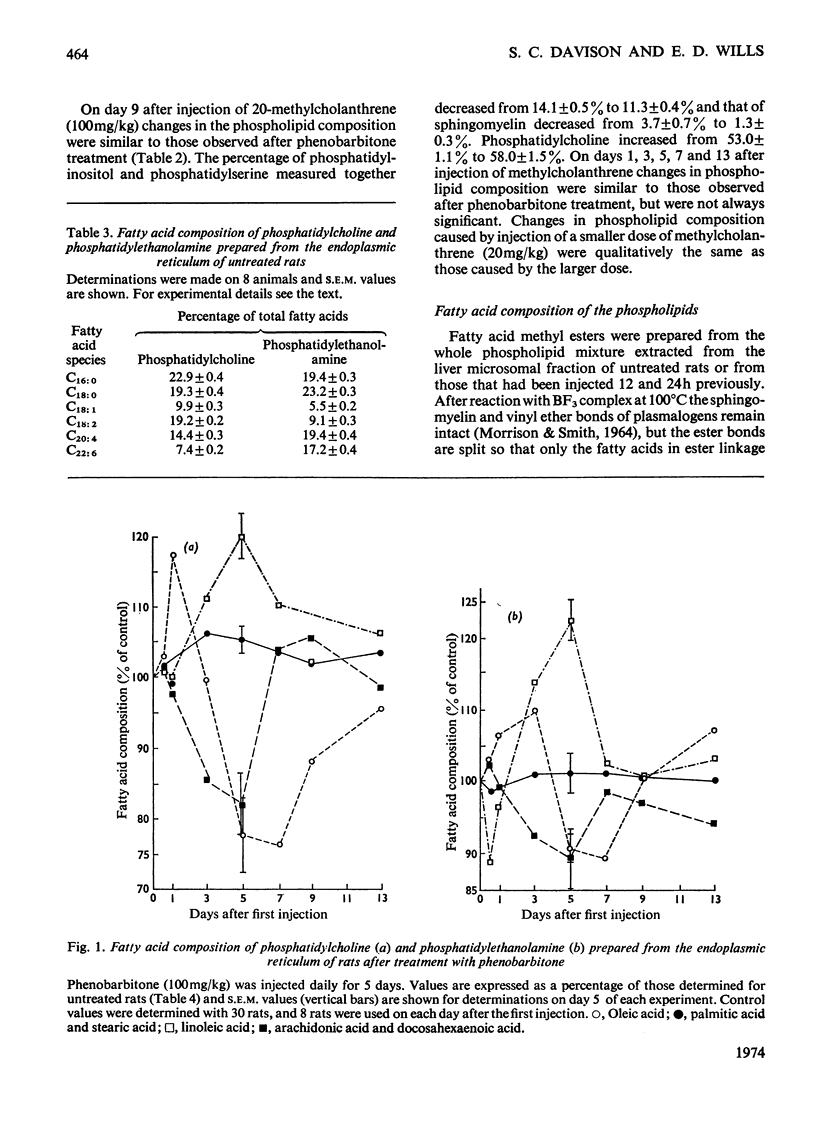
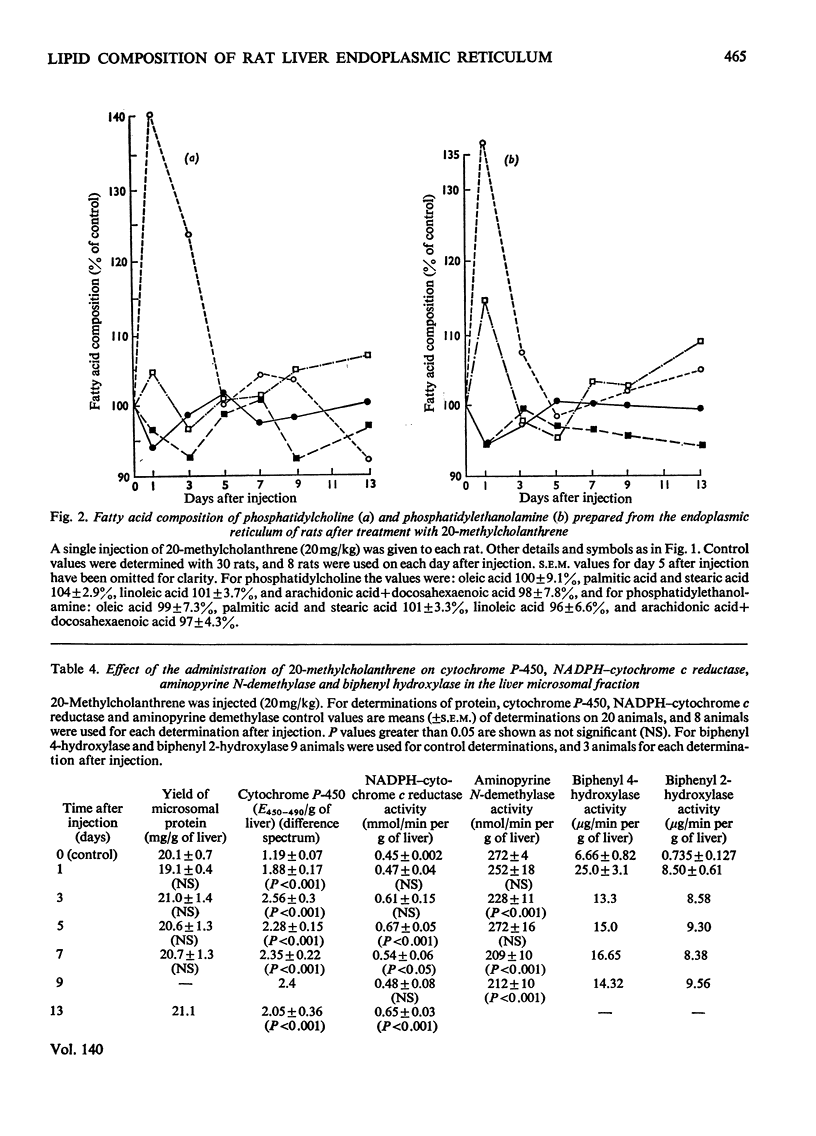
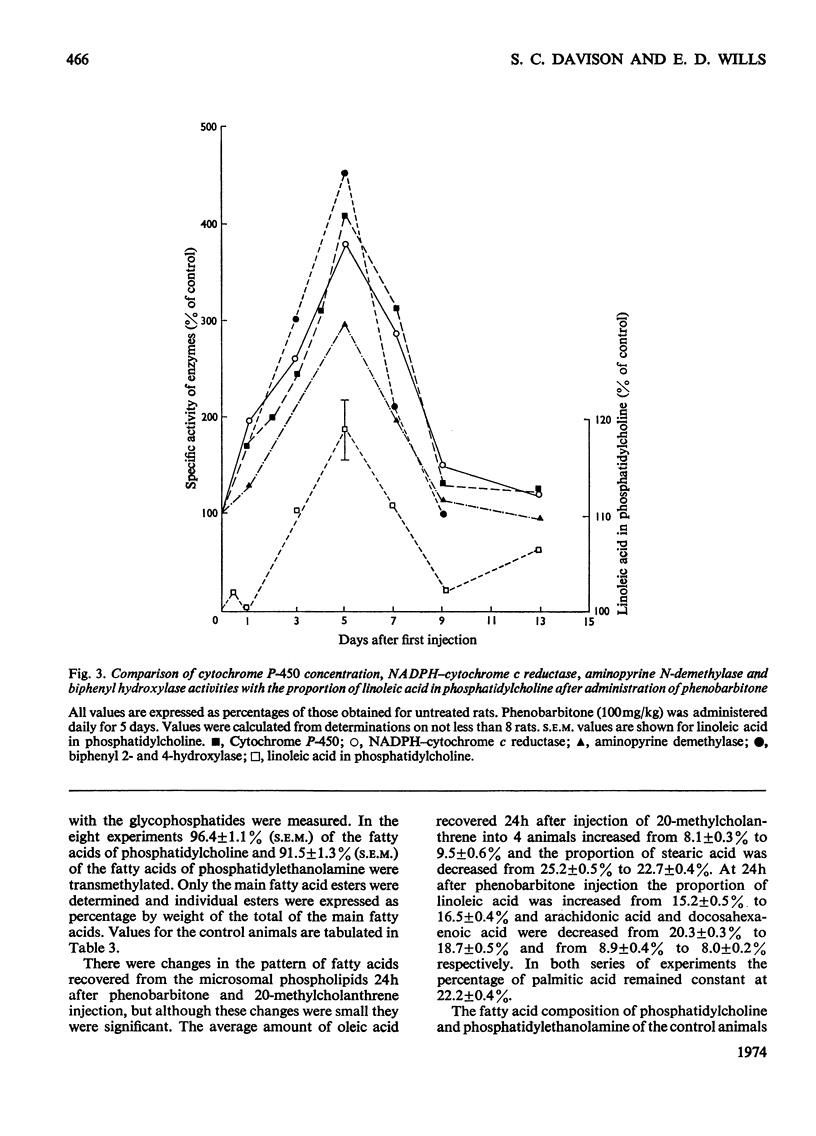
1. The cholesterol content, proportions of different phospholipids and fatty acid components of phosphatidylcholine and phosphatidylethanolamine were studied in rat liver endoplasmic-reticulum membrane, after a single injection of 20-methylcholanthrene or injections of phenobarbitone for 5 days. 2. A marked decrease in the proportion of cholesterol occurred 5 days after injection of 20-methylcholanthrene or phenobarbitone. 3. The proportion of phosphatidylcholine was increased by injection of phenobarbitone and minor changes occurred in other phospholipids. 4. Phenobarbitone caused the proportion of linoleic acid in phosphatidylcholine and phosphatidylethanolamine to increase to 120–125% of the control and the proportion of oleic acid, arachidonic acid and docosahexaenoic acid to decrease. 5. 20-Methylcholanthrene caused an increase in the proportion of oleic acid in phosphatidylcholine and ethanolamine to 125–140% of the control, 1 day after injection. 6. The increased proportion of linoleic acid in phosphatidylcholine after phenobarbitone injection occurs simultaneously with the increase of cytochrome P-450 concentration, the rate of oxidative demethylation of aminopyrine and the rate of hydroxylation of biphenyl. It is therefore considered that distinct species of phosphatidylcholine or phosphatidylethanolamine containing linoleic acid in the β position are essential in the endoplasmic-reticulum membrane for optimal activity of oxidative demethylation.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arias I. M., Doyle D., Schimke R. T. Studies on the synthesis and degradation of proteins of the endoplasmic reticulum of rat liver. J Biol Chem. 1969 Jun 25;244(12):3303–3315. [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Chaplin M. D., Mannering G. J. Role of phospholipids in the hepatic microsomal drug-metabolizing system. Mol Pharmacol. 1970 Nov;6(6):631–640. [PubMed] [Google Scholar]
- Colbeau A., Nachbaur J., Vignais P. M. Enzymic characterization and lipid composition of rat liver subcellular membranes. Biochim Biophys Acta. 1971 Dec 3;249(2):462–492. doi: 10.1016/0005-2736(71)90123-4. [DOI] [PubMed] [Google Scholar]
- Conney A. H. Pharmacological implications of microsomal enzyme induction. Pharmacol Rev. 1967 Sep;19(3):317–366. [PubMed] [Google Scholar]
- Creaven P. J., Parke D. V., Williams R. T. A fluorimetric study of the hydroxylation of biphenyl in vitro by liver preparations of various species. Biochem J. 1965 Sep;96(3):879–885. doi: 10.1042/bj0960879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodge J. T., Phillips G. B. Autoxidation as a cause of altered lipid distribution in extracts from human red cells. J Lipid Res. 1966 May;7(3):387–395. [PubMed] [Google Scholar]
- Eling T. E., DiAugustine R. P. A role for phospholipids in the binding and metabolism of drugs by hepatic microsomes. Use of the fluorescent hydrophobic probe 1-anilinonaphthalene-8-sulphonate. Biochem J. 1971 Jul;123(4):539–549. doi: 10.1042/bj1230539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FOUTS J. R., ROGERS L. A. MORPHOLOGICAL CHANGES IN THE LIVER ACCOMPANYING STIMULATION OF MICROSOMAL DRUG METABOLIZING ENZYME ACTIVITY BY PHENOBARBITAL, CHLORDANE, BENZPYRENE OR METHYL-CHOLANTHRENE IN RATS. J Pharmacol Exp Ther. 1965 Jan;147:112–119. [PubMed] [Google Scholar]
- Glaumann H., Dallner G. Lipid composition and turnover of rough and smooth microsomal membranes in rat liver. J Lipid Res. 1968 Nov;9(6):720–729. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lu A. Y., Junk K. W., Coon M. J. Resolution of the cytochrome P-450-containing omega-hydroxylation system of liver microsomes into three components. J Biol Chem. 1969 Jul 10;244(13):3714–3721. [PubMed] [Google Scholar]
- MANN G. V. A method for measurement of cholesterol in blood serum. Clin Chem. 1961 Jun;7:275–284. [PubMed] [Google Scholar]
- MORRISON W. R., SMITH L. M. PREPARATION OF FATTY ACID METHYL ESTERS AND DIMETHYLACETALS FROM LIPIDS WITH BORON FLUORIDE--METHANOL. J Lipid Res. 1964 Oct;5:600–608. [PubMed] [Google Scholar]
- OMURA T., SATO R. THE CARBON MONOXIDE-BINDING PIGMENT OF LIVER MICROSOMES. I. EVIDENCE FOR ITS HEMOPROTEIN NATURE. J Biol Chem. 1964 Jul;239:2370–2378. [PubMed] [Google Scholar]
- Orrenius S., Ericsson J. L., Ernster L. Phenobarbital-induced synthesis of the microsomal drug-metabolizing enzyme system and its relationship to the proliferation of endoplasmic membranes. A morphological and biochemical study. J Cell Biol. 1965 Jun;25(3):627–639. doi: 10.1083/jcb.25.3.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasternak C. A., Bergeron J. J. Turnover of mammalian phospholipids. Stable and unstable components in neoplastic mast cells. Biochem J. 1970 Sep;119(3):473–480. doi: 10.1042/bj1190473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REMMER H., MERKER H. J. DRUG-INDUCED CHANGES IN THE LIVER ENDOPLASMIC RETICULUM: ASSOCIATION WITH DRUG-METABOLIZING ENZYMES. Science. 1963 Dec 27;142(3600):1657–1658. doi: 10.1126/science.142.3600.1657. [DOI] [PubMed] [Google Scholar]
- Rouser G., Siakotos A. N., Fleischer S. Quantitative analysis of phospholipids by thin-layer chromatography and phosphorus analysis of spots. Lipids. 1966 Jan;1(1):85–86. doi: 10.1007/BF02668129. [DOI] [PubMed] [Google Scholar]
- Skipski V. P., Peterson R. F., Barclay M. Quantitative analysis of phospholipids by thin-layer chromatography. Biochem J. 1964 Feb;90(2):374–378. doi: 10.1042/bj0900374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strobel H. W., Lu A. Y., Heidema J., Coon M. J. Phosphatidylcholine requirement in the enzymatic reduction of hemoprotein P-450 and in fatty acid, hydrocarbon, and drug hydroxylation. J Biol Chem. 1970 Sep 25;245(18):4851–4854. [PubMed] [Google Scholar]
- WILLIAMS C. H., Jr, KAMIN H. Microsomal triphosphopyridine nucleotide-cytochrome c reductase of liver. J Biol Chem. 1962 Feb;237:587–595. [PubMed] [Google Scholar]
- Wills E. D. Effects of lipid peroxidation on membrane-bound enzymes of the endoplasmic reticulum. Biochem J. 1971 Aug;123(5):983–991. doi: 10.1042/bj1230983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wills E. D. Lipid peroxide formation in microsomes. Relationship of hydroxylation to lipid peroxide formation. Biochem J. 1969 Jun;113(2):333–341. doi: 10.1042/bj1130333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young D. L., Powell G., McMillan W. O. Phenobarbital-induced alterations in phosphatidylcholine and triglyceride synthesis in hepatic endoplasmic reticulum. J Lipid Res. 1971 Jan;12(1):1–8. [PubMed] [Google Scholar]


