Abstract
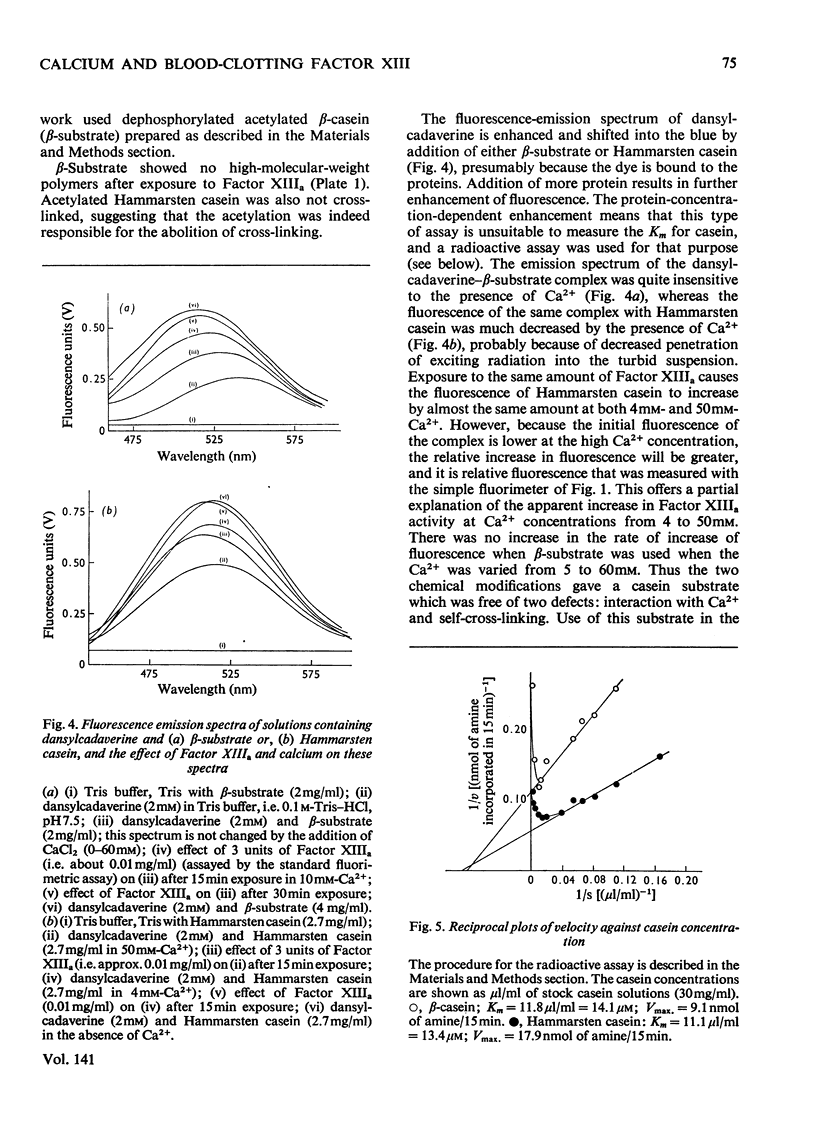
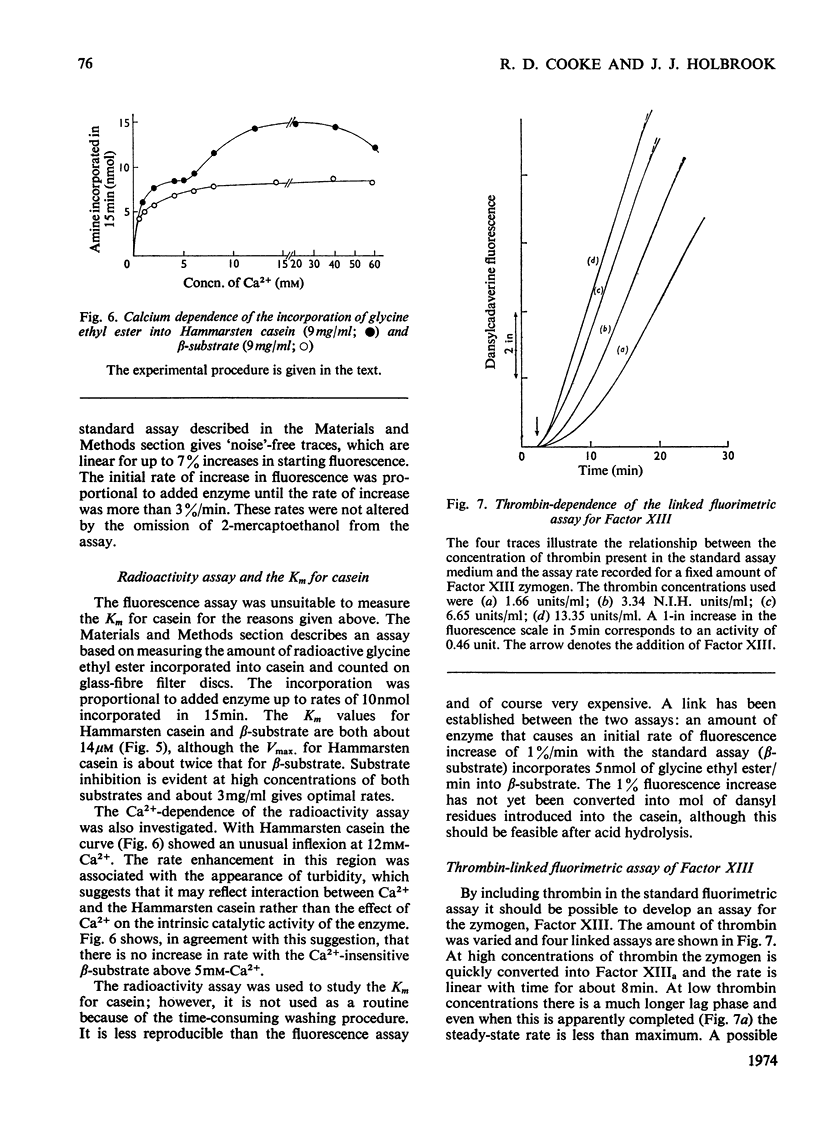
1. A continuous fluorimetric assay for blood-clotting Factor XIII based on the incorporation of dansylcadaverine into casein was investigated. 2. Hammarsten casein was fractionated to yield the β-casein, which was dephosphorylated and acetylated to give a substrate which itself did not bind calcium and which was not cross-linked by the activated Factor. 3. The modified β-casein was used as substrate for a continuous fluorimetric assay and as substrate for the incorporation of radioactive glycine ethylester. 4. A linked fluorimetric assay for the zymogen is described. 5. The Km for calcium was redetermined and at 0.2mm was in the physiological range and much lower than the values reported by others using substrates which interact with calcium. 6. The Km for casein is about 14μm.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BETTELHEIM F. R., BAILEY K. The products of the action of thrombin on fibrinogen. Biochim Biophys Acta. 1952 Nov;9(5):578–579. doi: 10.1016/0006-3002(52)90213-8. [DOI] [PubMed] [Google Scholar]
- Berg H. C. Sulfanilic acid diazonium salt: a label for the outside of the human erythrocyte membrane. Biochim Biophys Acta. 1969 Jun 3;183(1):65–78. doi: 10.1016/0005-2736(69)90130-8. [DOI] [PubMed] [Google Scholar]
- Bingham E. W., Farrell H. M., Jr, Carroll R. J. Properties of dephosphorylated s1 -casein. Precipitation by calcium ions and micelle formation. Biochemistry. 1972 Jun 20;11(13):2450–2454. doi: 10.1021/bi00763a010. [DOI] [PubMed] [Google Scholar]
- Bretscher M. S. Human erythrocyte membranes: specific labelling of surface proteins. J Mol Biol. 1971 Jun 28;58(3):775–781. doi: 10.1016/0022-2836(71)90039-8. [DOI] [PubMed] [Google Scholar]
- Chung S. I., Folk J. E. Kinetic studies with transglutaminases. The human blood enzymes (activated coagulation factor 13 and the guinea pig hair follicle enzyme. J Biol Chem. 1972 May 10;247(9):2798–2807. [PubMed] [Google Scholar]
- DUMAS B. R., MAUBOIS J. L., MOCQUOT G., GARNIER J. ETUDE DE LA CONSTITUTION DE LA CAS'EINE DE VACHE PAR CHROMATOGRAPHIE SUR COLONNES DE DIETHYLAMINOETHYL-CELLULOSE EN MILIEU UR'EE. Biochim Biophys Acta. 1964 Mar 16;82:494–506. [PubMed] [Google Scholar]
- Deranleau D. A., Neurath H. The combination of chymotrypsin and chymotrypsinogen with fluorescent substrates and inhibitors for chymotrypsin. Biochemistry. 1966 Apr;5(4):1413–1425. doi: 10.1021/bi00868a040. [DOI] [PubMed] [Google Scholar]
- Dvilansky A., Britten A. F., Loewy A. G. Factor XIII assay by an isotope method. I. Factor XIII (transamidase) in plasma, serum, leucocytes, erythrocytes and platelets and evaluation of screening tests of clot solubility. Br J Haematol. 1970 Apr;18(4):399–410. doi: 10.1111/j.1365-2141.1970.tb01453.x. [DOI] [PubMed] [Google Scholar]
- GORMSEN J., SIVERTSEN U. THE EFFECT OF SULFHYDRYLINHIBITORS AND GLYCINE DERIVATIVES ON FIBRIN POLYMERIZATION AND THE PHYSICAL STRENGTH OF FIBRIN IN PLASMA. Thromb Diath Haemorrh. 1964 Jul 31;11:454–467. [PubMed] [Google Scholar]
- Gray W. R., Del Valle U. E. Application of mass spectrometry to protein chemistry. I. Method for amino-terminal sequence analysis of proteins. Biochemistry. 1970 May 12;9(10):2134–2137. doi: 10.1021/bi00812a600. [DOI] [PubMed] [Google Scholar]
- Halford S. E., Bennett N. G., Trentham D. R., Gutfeund H. A substate-induced conformation change in the reaction of alkaline phosphatase from Escherichia coli. Biochem J. 1969 Sep;114(2):243–251. doi: 10.1042/bj1140243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LINDER E., BLOMSTRAND R. Technic for collection of thoracic duct lymph of man. Proc Soc Exp Biol Med. 1958 Mar;97(3):653–657. doi: 10.3181/00379727-97-23836. [DOI] [PubMed] [Google Scholar]
- LOEWY A. G., DUNATHAN K., KRIEL R., WOLFINGER H. L., Jr Fibrinase. I. Purification of substrate and enzyme. J Biol Chem. 1961 Oct;236:2625–2633. [PubMed] [Google Scholar]
- LORAND L., JACOBSEN A. SPECIFIC INHIBITORS AND THE CHEMISTRY OF FIBRIN POLYMERIZATION. Biochemistry. 1964 Dec;3:1939–1943. doi: 10.1021/bi00900a026. [DOI] [PubMed] [Google Scholar]
- LORAND L., KONISHI K. ACTIVATION OF THE FIBRIN STABILIZING FACTOR OF PLASMA BY THROMBIN. Arch Biochem Biophys. 1964 Apr;105:58–67. doi: 10.1016/0003-9861(64)90235-8. [DOI] [PubMed] [Google Scholar]
- LORAND L., KONISHI K., JACOBSEN A. Transpeptidation mechanism in blood clotting. Nature. 1962 Jun 23;194:1148–1149. doi: 10.1038/1941148a0. [DOI] [PubMed] [Google Scholar]
- Lorand L., Campbell-Wilkes L. K., Cooperstein L. A filter paper assay for transamidating enzymes using radioactive amine substrates. Anal Biochem. 1972 Dec;50(2):623–631. doi: 10.1016/0003-2697(72)90074-7. [DOI] [PubMed] [Google Scholar]
- Lorand L., Chou C. H., Simpson I. Thiolester substrates for transamidating enzymes: studies on fibrinoligase. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2645–2648. doi: 10.1073/pnas.69.9.2645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorand L., Downey J., Gotoh T., Jacobsen A., Tokura S. The transpeptidase system which crosslinks fibrin by gamma-glutamyle-episilon-lysine bonds. Biochem Biophys Res Commun. 1968 Apr 19;31(2):222–230. doi: 10.1016/0006-291x(68)90734-1. [DOI] [PubMed] [Google Scholar]
- Lorand L., Lockridge O. M., Campbell L. K., Myhrman R., Bruner-Lorand J. Transamidating enzymes. II. A continuous fluorescent method suited for automating measurements of factor XIII in plasma. Anal Biochem. 1971 Nov;44(1):221–231. doi: 10.1016/0003-2697(71)90363-0. [DOI] [PubMed] [Google Scholar]
- Lorand L., Ong H. H. Labeling of amine-acceptor cross-linking sites of fibrin by transpeptidation. Biochemistry. 1966 May;5(5):1747–1753. doi: 10.1021/bi00869a043. [DOI] [PubMed] [Google Scholar]
- Lorand L., Urayama T., De Kiewiet J. W., Nossel H. L. Diagnostic and genetic studies on fibrin-stabilizing factor with a new assay based on amine incorporation. J Clin Invest. 1969 Jun;48(6):1054–1064. doi: 10.1172/JCI106061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matacić S., Loewy A. G. Transglutaminase activity of the fibrin crosslinking enzyme. Biochem Biophys Res Commun. 1966 Sep 22;24(6):858–866. doi: 10.1016/0006-291x(66)90327-5. [DOI] [PubMed] [Google Scholar]
- Pisano J. J., Finlayson J. S., Peyton M. P. [Cross-link in fibrin polymerized by factor 13: epsilon-(gamma-glutamyl)lysine]. Science. 1968 May 24;160(3830):892–893. doi: 10.1126/science.160.3830.892. [DOI] [PubMed] [Google Scholar]
- Schwartz M. L., Pizzo S. V., Hill R. L., McKee P. A. The effect of fibrin-stabilizing factor on the subunit structure of human fibrin. J Clin Invest. 1971 Jul;50(7):1506–1513. doi: 10.1172/JCI106636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro A. L., Viñuela E., Maizel J. V., Jr Molecular weight estimation of polypeptide chains by electrophoresis in SDS-polyacrylamide gels. Biochem Biophys Res Commun. 1967 Sep 7;28(5):815–820. doi: 10.1016/0006-291x(67)90391-9. [DOI] [PubMed] [Google Scholar]
- Sheltawy M. J., Miloszewski K., Losowsky M. S. Factors affecting factor XIII assay by dansyl cadaverine incorporation. Thromb Diath Haemorrh. 1972 Dec 31;28(3):483–488. [PubMed] [Google Scholar]
- Tanner M. J., Boxer D. H. Separation and some properties of the major proteins of the human erythrocyte membrane. Biochem J. 1972 Sep;129(2):333–347. doi: 10.1042/bj1290333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler H. M. Fibrin crosslinking demonstrated by thrombelastography. Thromb Diath Haemorrh. 1969 Nov 15;22(2):398–400. [PubMed] [Google Scholar]
- Waugh D. F., Creamer L. K., Slattery C. W., Dresdner G. W. Core polymers of casein micelles. Biochemistry. 1970 Feb 17;9(4):786–795. doi: 10.1021/bi00806a011. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]