Abstract
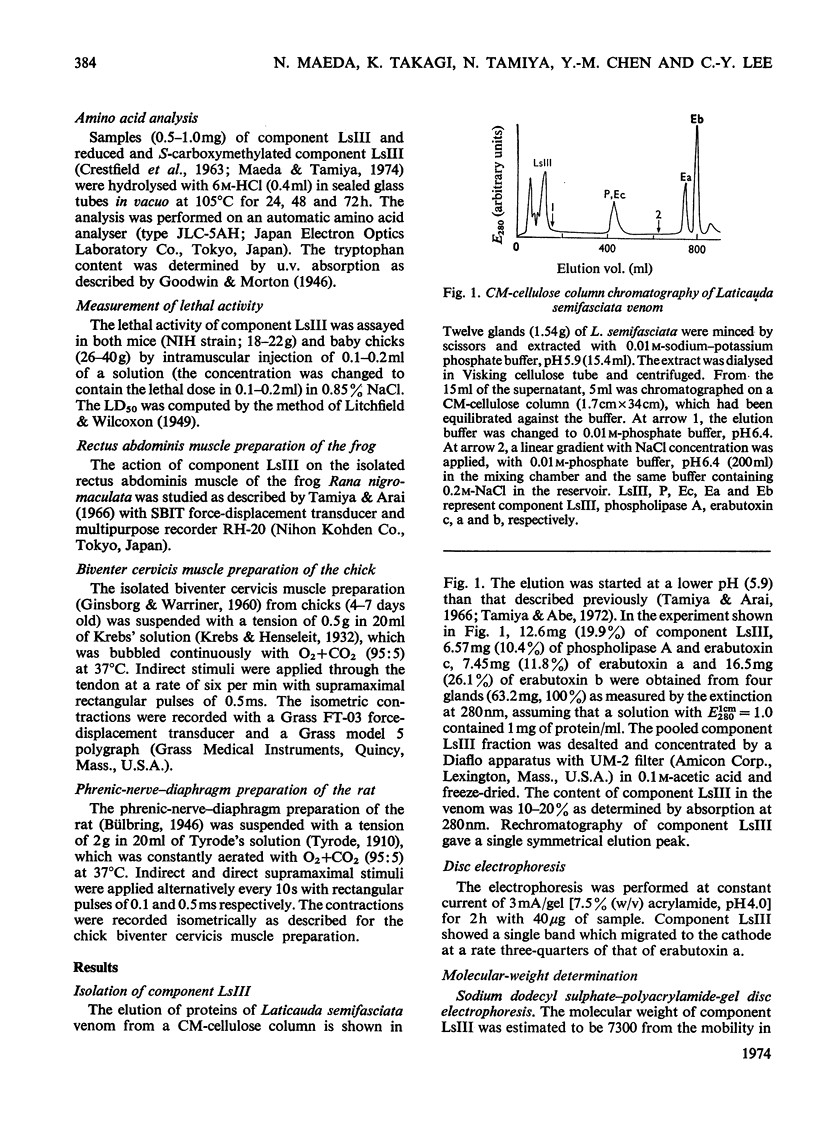
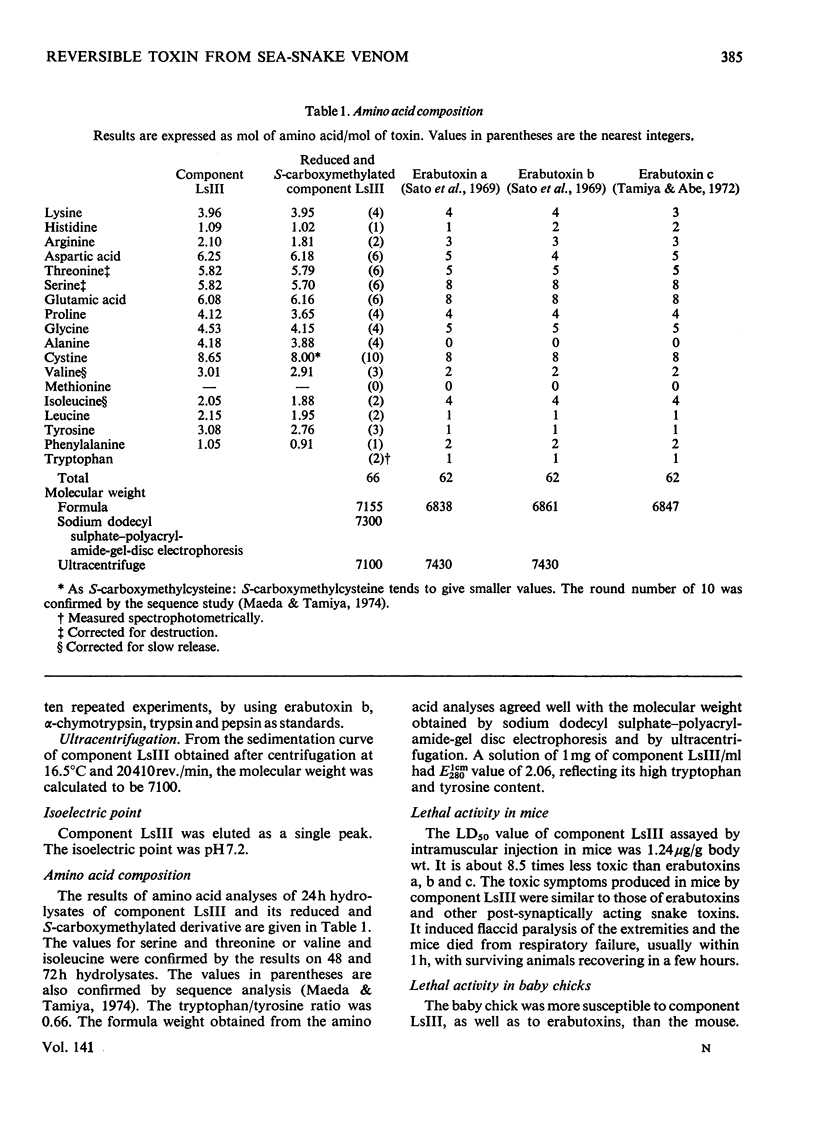
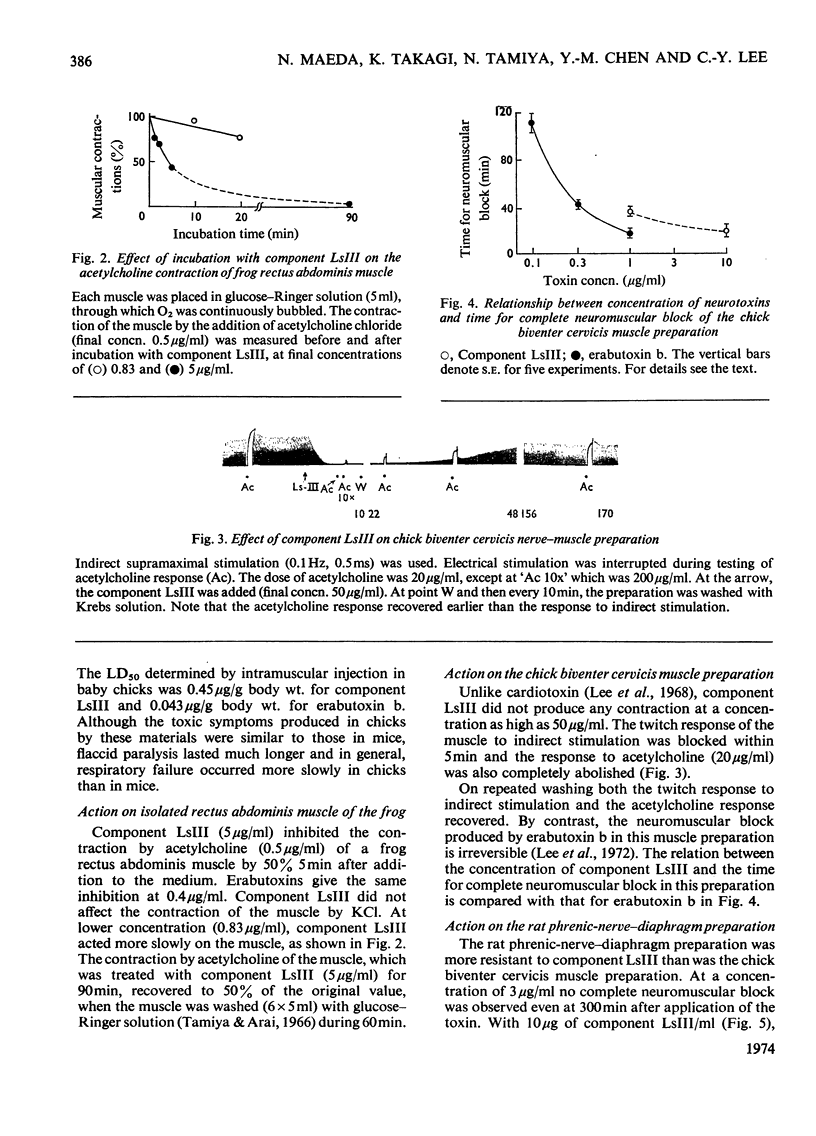
A weakly neurotoxic component (Ls-III) was isolated by CM-cellulose column chromatography from the venom of a sea snake Laticauda semifasciata. The content of component LsIII was about 10–20% of the venom as determined by u.v. absorption at 280nm. Component LsIII was homogeneous on rechromatography and disc electrophoresis, and its molecular weight was shown to be 7100 by ultracentrifugation and 7300 by sodium dodecyl sulphate–polyacrylamide-gel disc electrophoresis. The isoelectric point of component LsIII was pH7.2. Component LsIII consisted of 66 amino acid residues including 10 half-cystine residues. The LD50 of component LsIII by intramuscular injection was 1.24μg/g body wt. for mice and 0.45μg/g for baby chicks, which is about eight to ten times less toxic than erabutoxins a, b and c, all of which are contained in the same venom. Experiments with three isolated muscle preparations from different species indicated that component LsIII was a post-synaptically acting toxin, the action of which was easily reversed by washing.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CRESTFIELD A. M., MOORE S., STEIN W. H. The preparation and enzymatic hydrolysis of reduced and S-carboxymethylated proteins. J Biol Chem. 1963 Feb;238:622–627. [PubMed] [Google Scholar]
- Endo Y., Sato S., Ishii S., Tamiya N. The disulphide bonds of erabutoxin a, a neurotoxic protein of a sea-snake (Laticauda semifasciata) venom. Biochem J. 1971 May;122(4):463–467. doi: 10.1042/bj1220463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GINSBORG B. L., WARRINER J. The isolated chick biventer cervicis nerve-muscle preparation. Br J Pharmacol Chemother. 1960 Sep;15:410–411. doi: 10.1111/j.1476-5381.1960.tb01264.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin T. W., Morton R. A. The spectrophotometric determination of tyrosine and tryptophan in proteins. Biochem J. 1946;40(5-6):628–632. doi: 10.1042/bj0400628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C. Y., Chang C. C., Chen Y. M. Reversibility of neuromuscular blockade by neurotoxins from elapid and sea snake venoms. Taiwan Yi Xue Hui Za Zhi. 1972 Jun 28;71(6):344–349. [PubMed] [Google Scholar]
- Lee C. Y., Chang C. C., Chiu T. H., Chiu P. J., Tseng T. C., Lee S. Y. Pharmacological properties of cardiotoxin isolated from Formosan cobra venom. Naunyn Schmiedebergs Arch Exp Pathol Pharmakol. 1968;259(4):360–374. doi: 10.1007/BF00536909. [DOI] [PubMed] [Google Scholar]
- Lee C. Y. Chemistry and pharmacology of polypeptide toxins in snake venoms. Annu Rev Pharmacol. 1972;12:265–286. doi: 10.1146/annurev.pa.12.040172.001405. [DOI] [PubMed] [Google Scholar]
- Maeda N., Tamiya N. The primary structure of the toxin Laticauda semifasciata III, a weak and reversibly acting neurotoxin from the venom of a sea snake, Laticauda semifasciata. Biochem J. 1974 Aug;141(2):389–400. doi: 10.1042/bj1410389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REISFELD R. A., LEWIS U. J., WILLIAMS D. E. Disk electrophoresis of basic proteins and peptides on polyacrylamide gels. Nature. 1962 Jul 21;195:281–283. doi: 10.1038/195281a0. [DOI] [PubMed] [Google Scholar]
- Sato S., Tamiya N. The amino acid sequences of erabutoxins, neurotoxic proteins of sea-snake (Laticauda semifasciata) venom. Biochem J. 1971 May;122(4):453–461. doi: 10.1042/bj1220453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato S., Yoshida H., Abe H., Tamiya N. Properties and biosynthesis of a neurotoxic protein of the venoms of s snakes Laticauda laticaudata and Laticauda colubrina. Biochem J. 1969 Oct;115(1):85–90. doi: 10.1042/bj1150085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strydom D. J. Snake venom toxins. Structure-function relationships and phylogenetics. Comp Biochem Physiol B. 1973 Jan 15;44(1):269–281. doi: 10.1016/0305-0491(73)90364-7. [DOI] [PubMed] [Google Scholar]
- Tamiya N., Abe H. The isolation, properties and amino acid sequence of erabutoxin c, a minor neurotoxic component of the venom of a sea snake Katicauda semifasciata. Biochem J. 1972 Nov;130(2):547–555. doi: 10.1042/bj1300547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamiya N., Arai H. Studies on sea-snake venoms. Crystallization of erabutoxins a and b from Laticauda semifasciata venom. Biochem J. 1966 Jun;99(3):624–630. doi: 10.1042/bj0990624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- YPHANSTIS D. A. Rapid determination of molecular weights of peptides and preteins. Ann N Y Acad Sci. 1960 Aug 31;88:586–601. doi: 10.1111/j.1749-6632.1960.tb20055.x. [DOI] [PubMed] [Google Scholar]