Abstract
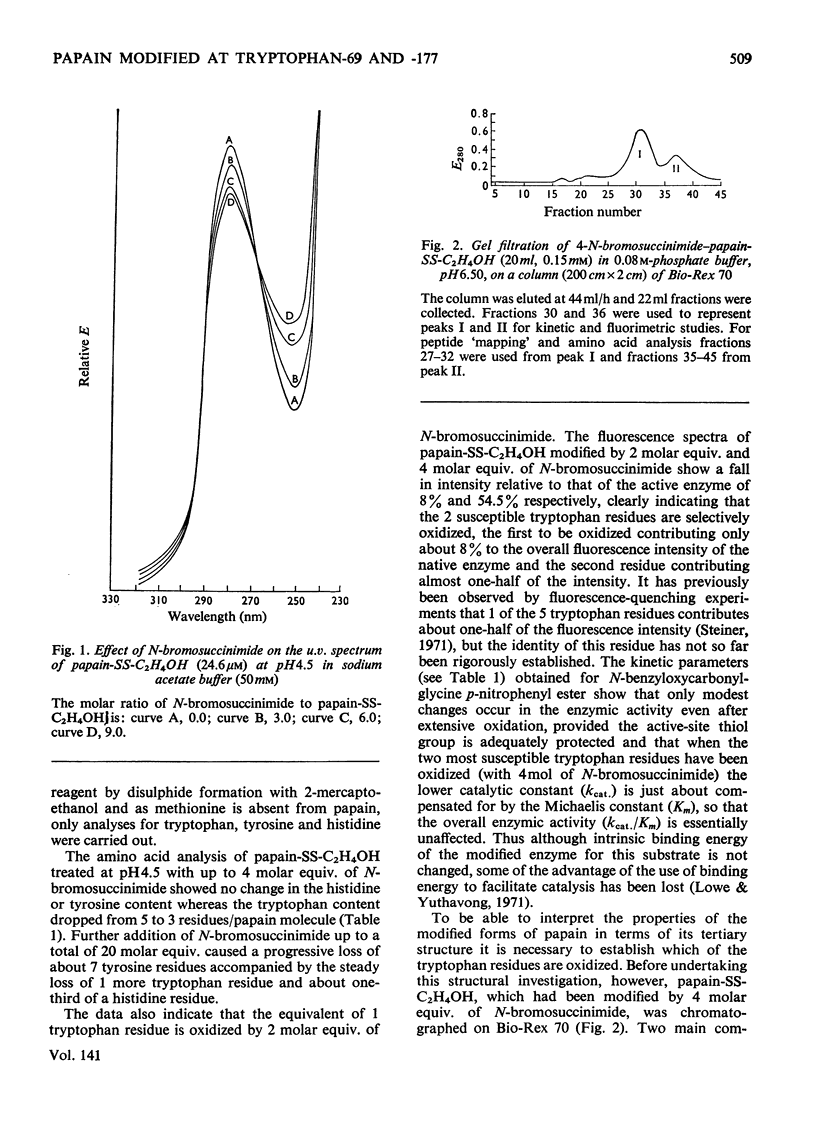
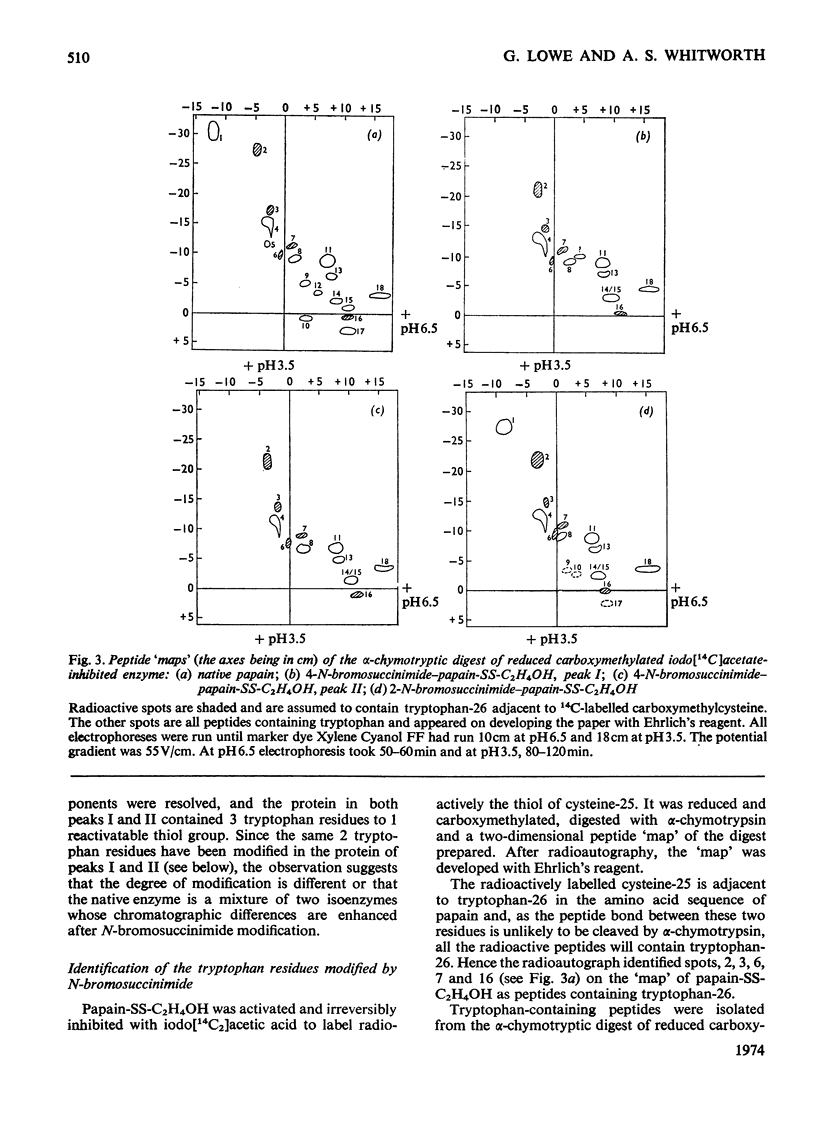
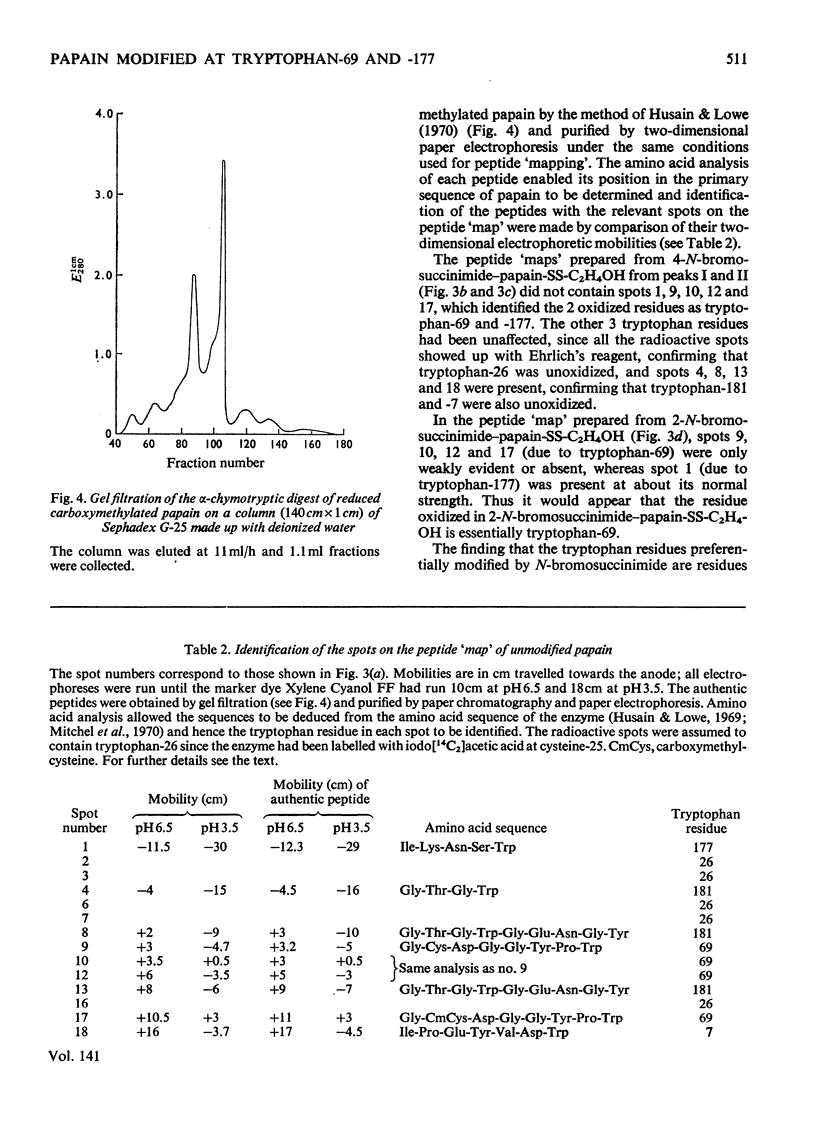
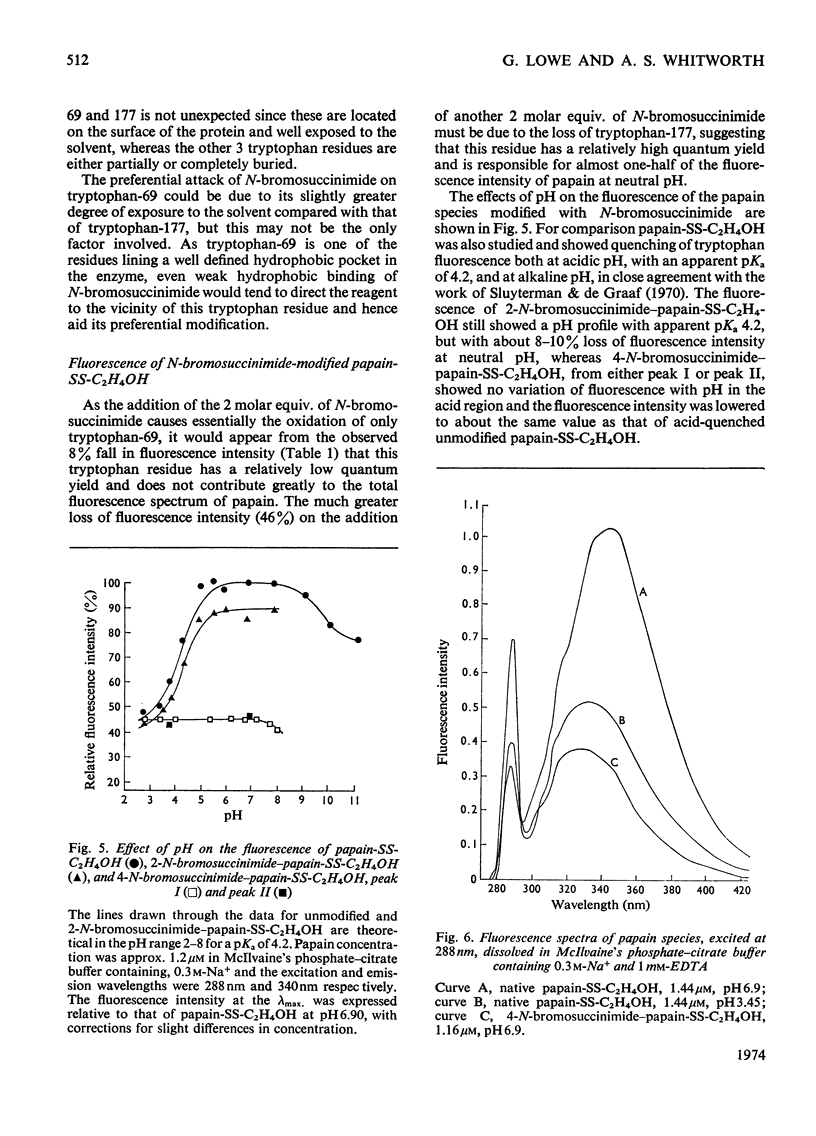
A systematic study of the modification of papain (its thiol group protected as a disulphide with mercaptoethanol) by N-bromosuccinimide, showed that 2 molar equiv. modified tryptophan-69 and 4 molar equiv. modified tryptophan-69 and -177. The Michaelis parameters for the catalysed hydrolysis of N-benzyloxycarbonylglycine p-nitrophenyl ester by these modified enzymes were determined. The enzymic activity of the modified enzymes was not seriously impaired, but modification of tryptophan-177 raised the apparent pKa of the acidic limb of the pH profile by more than 1 pH unit for both kcat. and kcat./Km. The fluorescence spectra (excitation at 288nm) of the modified enzymes showed that tryptophan-69 contributed about 8% to the fluorescence intensity, whereas tryptophan-177 contributed about 46% at neutral pH. However, the contribution of tryptophan-177 was quenched at low pH and its fluorescence intensity showed sigmoidal pH-dependence, with an apparent pKa of 4.2. Histidine-159, which is in close contact with tryptophan-177, is considered to be the residue responsible for the fluorescence quenching. When tryptophan-177 was modified, presumably generating a less hydrophobic micro-environment, the apparent pKa determined kinetically was raised to about 5.4. By comparing the Michaelis parameters of native papain, papain modified at tryptophan-69 and papain modified at tryptophan-69 and -177 with N-benzyloxycarbonylglycylglycine amide and N-benzyloxycarbonylglycyltryptophan amide, tryptophan-69 and tryptophan-177 were shown to be structural features of the S2 and S1′ subsites respectively.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alecio M. R., Dann M. L., Lowe G. The specificity of the S1' subsite of papain. Biochem J. 1974 Aug;141(2):495–501. doi: 10.1042/bj1410495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen G., Lowe G. Investigation of the active site of papain with fluorescent probes. Biochem J. 1973 Aug;133(4):679–686. doi: 10.1042/bj1330679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger A., Schechter I. Mapping the active site of papain with the aid of peptide substrates and inhibitors. Philos Trans R Soc Lond B Biol Sci. 1970 Feb 12;257(813):249–264. doi: 10.1098/rstb.1970.0024. [DOI] [PubMed] [Google Scholar]
- Blumberg S., Schechter I., Berger A. The purification of papain by affinity chromatography. Eur J Biochem. 1970 Jul;15(1):97–102. doi: 10.1111/j.1432-1033.1970.tb00981.x. [DOI] [PubMed] [Google Scholar]
- ELLMAN G. L. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959 May;82(1):70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- GLAZER A. N., SMITH E. L. Phenolic hydroxyl ionization in papain. J Biol Chem. 1961 Nov;236:2948–2951. [PubMed] [Google Scholar]
- Husain S. S., Lowe G. A reinvestigation of residues 64-68 and 175 in papain. Evidence that residues 64 and 175 are asparagine. Biochem J. 1970 Feb;116(4):689–692. doi: 10.1042/bj1160689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husain S. S., Lowe G. Completion of the amino acid sequence of papain. Biochem J. 1969 Sep;114(2):279–288. doi: 10.1042/bj1140279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husain S. S., Lowe G. Evidence for histidine in the active site of papain. Biochem J. 1968 Aug;108(5):855–859. doi: 10.1042/bj1080855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jori G., Galiazzo G. Proflavine-sensitized selective photooxidation of the tryptophyl residues in papain. Photochem Photobiol. 1971 Nov;14(5):607–619. doi: 10.1111/j.1751-1097.1971.tb06200.x. [DOI] [PubMed] [Google Scholar]
- KIMMEL J. R., SMITH E. L. Crystalline papain. I. Preparation, specificity, and activation. J Biol Chem. 1954 Apr;207(2):515–531. [PubMed] [Google Scholar]
- Kirschenbaum D. M. The enhancement of the enzymatic activity of papain by reaction with N-bromosuccinimide. Biochim Biophys Acta. 1971 Apr 14;235(1):159–163. doi: 10.1016/0005-2744(71)90043-x. [DOI] [PubMed] [Google Scholar]
- Kronman M. J., Robbins F. M., Andreotti R. E. Reaction of N-bromosuccinimide with lysozyme. Biochim Biophys Acta. 1967 Dec 12;147(3):462–472. doi: 10.1016/0005-2795(67)90006-2. [DOI] [PubMed] [Google Scholar]
- Liu T. Y., Chang Y. H. Hydrolysis of proteins with p-toluenesulfonic acid. Determination of tryptophan. J Biol Chem. 1971 May 10;246(9):2842–2848. [PubMed] [Google Scholar]
- Lowe G., Yuthavong Y. Kinetic specificity in papain-catalysed hydrolyses. Biochem J. 1971 Aug;124(1):107–115. doi: 10.1042/bj1240107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Löffler H. G., Schneider F. Kinetische und proteinchemische Untersuchungen am Succinyl-Papain. Z Naturforsch B. 1972 Dec;27(12):1490–1497. [PubMed] [Google Scholar]
- Matsubara H., Sasaki R. M. High recovery of tryptophan from acid hydrolysates of proteins. Biochem Biophys Res Commun. 1969 Apr 29;35(2):175–181. doi: 10.1016/0006-291x(69)90263-0. [DOI] [PubMed] [Google Scholar]
- Mitchel R. E., Chaiken I. M., Smith E. L. The complete amino acid sequence of papain. Additions and corrections. J Biol Chem. 1970 Jul 25;245(14):3485–3492. [PubMed] [Google Scholar]
- Polgár L. On the mode of activation of the catalytically essential sulfhydryl group of papain. Eur J Biochem. 1973 Feb 15;33(1):104–109. doi: 10.1111/j.1432-1033.1973.tb02660.x. [DOI] [PubMed] [Google Scholar]
- SUN Y. K., TSOU C. L. STUDIES ON PAPAIN. III. ESSENTIAL HISTIDINE AND TRYPTOPHAN GROUPS. Sci Sin. 1963 Jun;12:879–884. [PubMed] [Google Scholar]
- Sluyterman L. A., Wijdenes J. An agarose mercurial column for the separation of mercaptopapain and nonmercaptopapain. Biochim Biophys Acta. 1970 Mar 31;200(3):593–595. doi: 10.1016/0005-2795(70)90122-4. [DOI] [PubMed] [Google Scholar]
- Sluyterman L. A., Wijdenes J. Cyanuration of papain. Activity and fluorescence of the products. Biochim Biophys Acta. 1972 Apr 15;263(2):329–338. [PubMed] [Google Scholar]
- Sluyterman L. A., de Graaf M. J. The fluorescence of papain. Biochim Biophys Acta. 1970 Mar 31;200(3):595–597. doi: 10.1016/0005-2795(70)90123-6. [DOI] [PubMed] [Google Scholar]
- Steiner R. F. Varying luminescence behavior of the different tryptophan residues of papain. Biochemistry. 1971 Mar 2;10(5):771–778. doi: 10.1021/bi00781a008. [DOI] [PubMed] [Google Scholar]
- WILKINSON G. N. Statistical estimations in enzyme kinetics. Biochem J. 1961 Aug;80:324–332. doi: 10.1042/bj0800324. [DOI] [PMC free article] [PubMed] [Google Scholar]


