Abstract
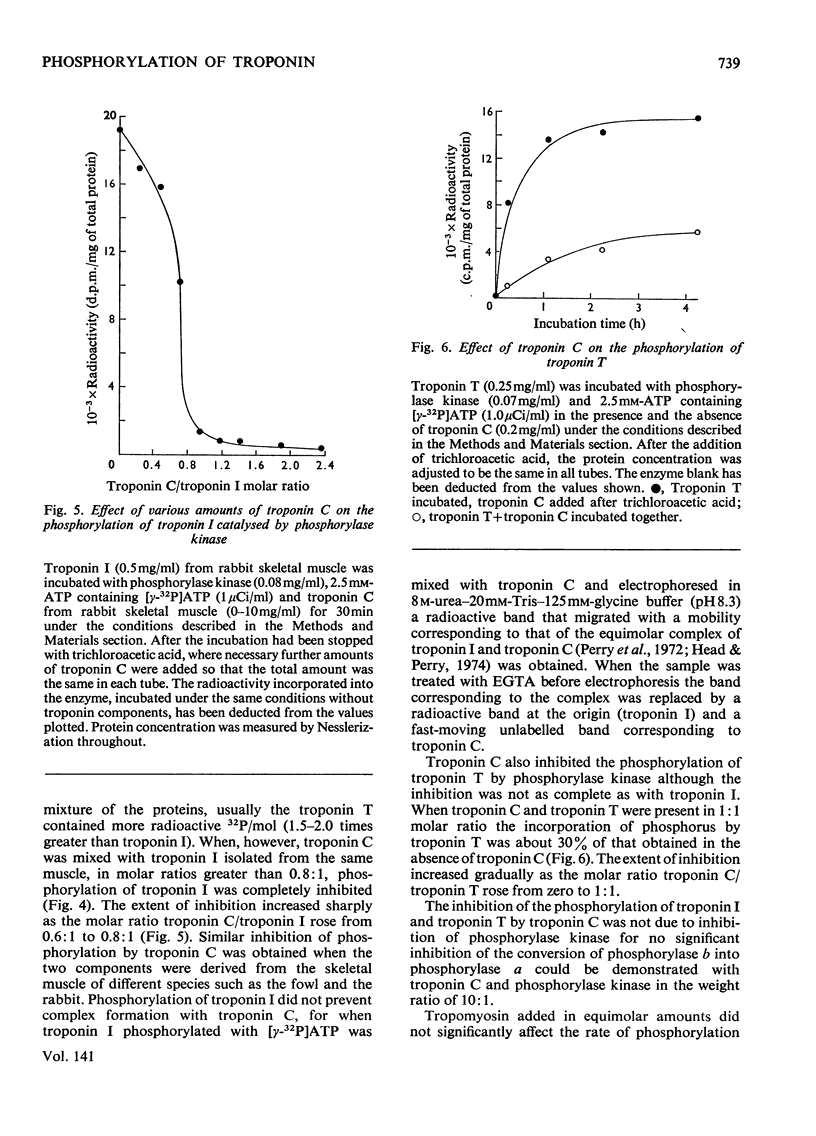
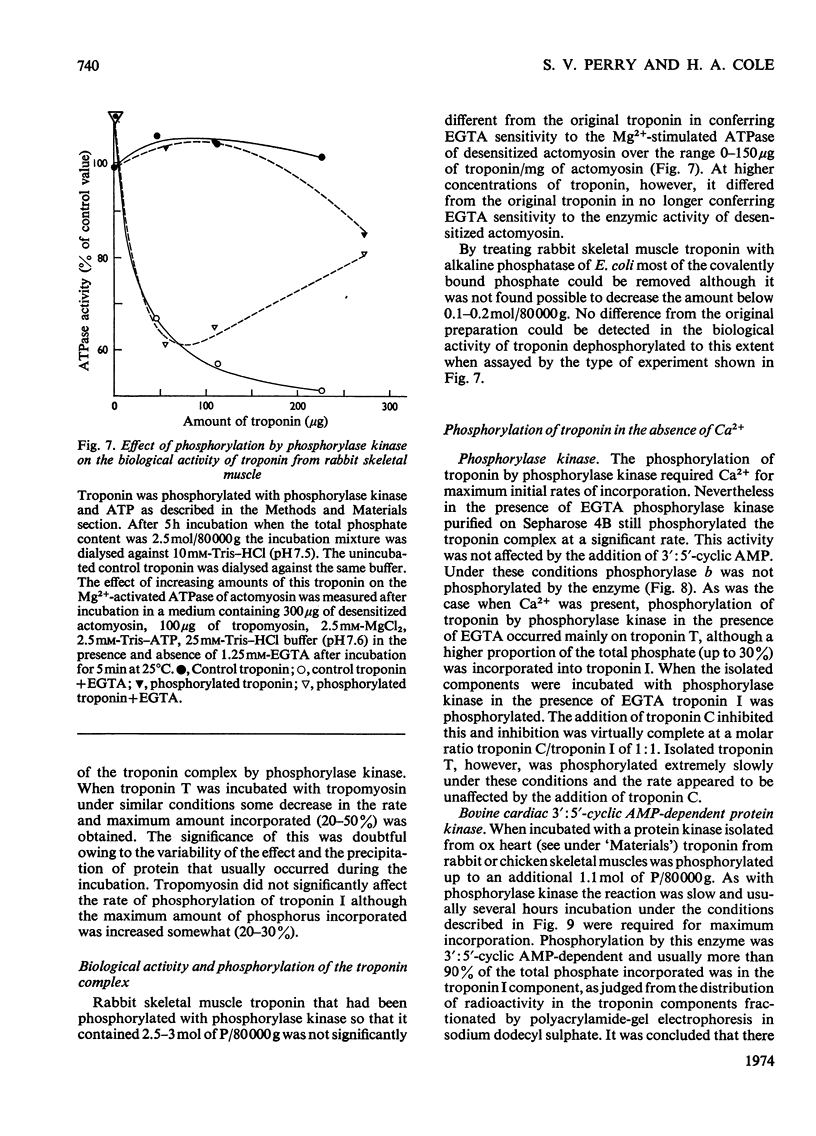
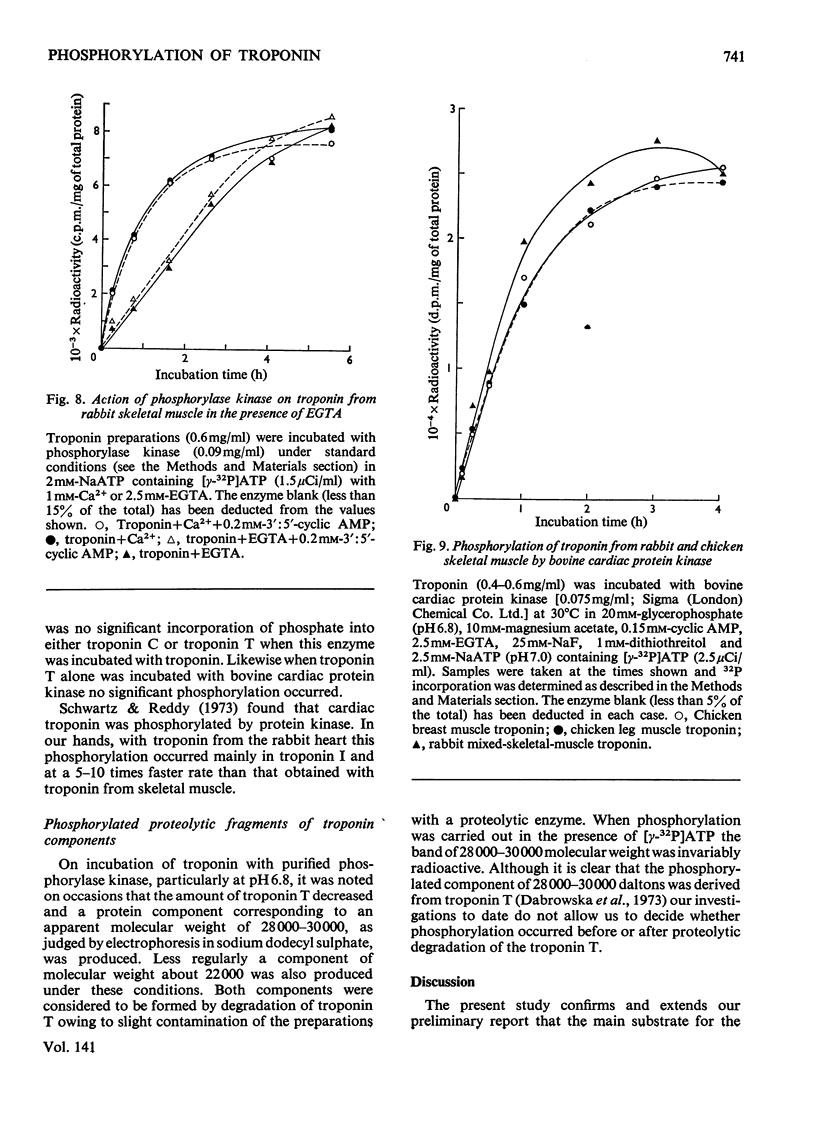
1. The troponin complex from skeletal muscle contains approximately 1 mol of phosphate/80000g of complex, covalently bound to the troponin T component. 2. On prolonged incubation of the troponin complex or troponin T with phosphorylase kinase the phosphate content of troponin T was increased to approx. 3mol/mol. 3. On prolonged incubation of troponin I with phosphorylase kinase up to 1.6mol of phosphate/mol were incorporated. 4. Phosphorylation of troponin I was greatly inhibited by troponin C owing to the strong interaction between these proteins. Thus in the troponin complex troponin T was the main substrate for phosphorylase kinase. The phosphorylation of isolated troponin T was also inhibited by troponin C. 5. Troponin I was phosphorylated when the troponin complex was incubated with a bovine cardiac 3′:5′-cyclic AMP-dependent protein kinase. Troponin T either in its isolated form or in the troponin complex was not phosphorylated by bovine protein kinase to any significant extent under the conditions used. 6. If the troponin complex was dephosphorylated to 0.2mol/mol, or phosphorylated up to 2.5mol/mol there was no significant effect on the ability of normal concentrations to confer Ca2+ sensitivity on the adenosine triphosphatase of densensitized actomyosin.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARTLETT G. R. Phosphorus assay in column chromatography. J Biol Chem. 1959 Mar;234(3):466–468. [PubMed] [Google Scholar]
- Barrett A. J., Dingle J. T. The inhibition of tissue acid proteinases by pepstatin. Biochem J. 1972 Apr;127(2):439–441. doi: 10.1042/bj1270439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brostrom C. O., Hunkeler F. L., Krebs E. G. The regulation of skeletal muscle phosphorylase kinase by Ca2+. J Biol Chem. 1971 Apr 10;246(7):1961–1967. [PubMed] [Google Scholar]
- Cohen P. The subunit structure of rabbit-skeletal-muscle phosphorylase kinase, and the molecular basis of its activation reactions. Eur J Biochem. 1973 Apr 2;34(1):1–14. doi: 10.1111/j.1432-1033.1973.tb02721.x. [DOI] [PubMed] [Google Scholar]
- Cummins P., Perry S. V. The subunits and biological activity of polymorphic forms of tropomyosin. Biochem J. 1973 Aug;133(4):765–777. doi: 10.1042/bj1330765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dabrowska R., Barylko B., Nowak E., Drabikowski W. The origin of 30,000 dalton protein in troponin preparations. FEBS Lett. 1973 Feb 1;29(3):239–242. doi: 10.1016/0014-5793(73)80028-6. [DOI] [PubMed] [Google Scholar]
- Ebashi S., Wakabayashi T., Ebashi F. Troponin and its components. J Biochem. 1971 Feb;69(2):441–445. doi: 10.1093/oxfordjournals.jbchem.a129486. [DOI] [PubMed] [Google Scholar]
- GAREN A., LEVINTHAL C. A fine-structure genetic and chemical study of the enzyme alkaline phosphatase of E. coli. I. Purification and characterization of alkaline phosphatase. Biochim Biophys Acta. 1960 Mar 11;38:470–483. doi: 10.1016/0006-3002(60)91282-8. [DOI] [PubMed] [Google Scholar]
- Gould J. M., Cather R., Winget G. D. Advantages of the use of Cerenkov vounting for determination of P 32 in photophosphorylation research. Anal Biochem. 1972 Dec;50(2):540–548. doi: 10.1016/0003-2697(72)90064-4. [DOI] [PubMed] [Google Scholar]
- Greaser M. L., Gergely J. Reconstitution of troponin activity from three protein components. J Biol Chem. 1971 Jul 10;246(13):4226–4233. [PubMed] [Google Scholar]
- Gross S. R., Mayer S. E. The phosphorylation of troponin B by phosphorylase b kinase in skeletal muscle of mice carrying the phosphorylase b kinase deficiency gene. Biochem Biophys Res Commun. 1973 Sep 18;54(2):823–830. doi: 10.1016/0006-291x(73)91498-8. [DOI] [PubMed] [Google Scholar]
- Hartshorne D. J., Mueller H. Fractionation of troponin into two distinct proteins. Biochem Biophys Res Commun. 1968 Jun 10;31(5):647–653. doi: 10.1016/0006-291x(68)90610-4. [DOI] [PubMed] [Google Scholar]
- Head J. F., Perry S. V. The interaction of the calcium-binding protein (troponin C) with bivalent cations and the inhibitory protein (troponin I). Biochem J. 1974 Feb;137(2):145–154. doi: 10.1042/bj1370145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitchcock S. E. Regulation of muscle contraction. Effect of calcium on the affinity of troponin for actin and tropomyosin. Biochemistry. 1973 Jun 19;12(13):2509–2513. doi: 10.1021/bi00737a022. [DOI] [PubMed] [Google Scholar]
- KREBS E. G., LOVE D. S., BRATVOLD G. E., TRAYSER K. A., MEYER W. L., FISCHER E. H. PURIFICATION AND PROPERTIES OF RABBIT SKELETAL MUSCLE PHOSPHORYLASE B KINASE. Biochemistry. 1964 Aug;3:1022–1033. doi: 10.1021/bi00896a003. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Perrie W. T., Smillie L. B., Perry S. B. A phosphorylated light-chain component of myosin from skeletal muscle. Biochem J. 1973 Sep;135(1):151–164. doi: 10.1042/bj1350151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry S. V., Cole H. A. Phosphorylation of the "37000 component" of the troponin complex (troponin-t). Biochem J. 1973 Feb;131(2):425–428. doi: 10.1042/bj1310425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry S. V. The control of muscular contraction. Symp Soc Exp Biol. 1973;27:531–550. [PubMed] [Google Scholar]
- Pires E., Perry S. V., Thomas M. A. Myosin light-chain kinase, a new enzyme from striated muscle. FEBS Lett. 1974 May 1;41(2):292–296. doi: 10.1016/0014-5793(74)81232-9. [DOI] [PubMed] [Google Scholar]
- Pratje E., Heilmeyer L. M.G. Phosphorylation of rabbit muscle troponin and actin by a 3', 5'-c-AMP-dependent protein kinase. FEBS Lett. 1972 Oct 15;27(1):89–93. doi: 10.1016/0014-5793(72)80416-2. [DOI] [PubMed] [Google Scholar]
- Schaub M. C., Perry S. V., Häcker W. The regulatory proteins of the myofibril. Characterization and biological activity of the calcium-sensitizing factor (troponin A). Biochem J. 1972 Jan;126(1):237–249. doi: 10.1042/bj1260237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaub M. C., Perry S. V. The relaxing protein system of striated muscle. Resolution of the troponin complex into inhibitory and calcium ion-sensitizing factors and their relationship to tropomyosin. Biochem J. 1969 Dec;115(5):993–1004. doi: 10.1042/bj1150993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stull J. T., Brostrom C. O., Krebs E. G. Phosphorylation of the inhibitor component of troponin by phosphorylase kinase. J Biol Chem. 1972 Aug 25;247(16):5272–5274. [PubMed] [Google Scholar]
- Tsukui R., Ebashi S. Cardiac troponin. J Biochem. 1973 May;73(5):1119–1121. doi: 10.1093/oxfordjournals.jbchem.a130168. [DOI] [PubMed] [Google Scholar]
- Van Eerd J. P., Kawasaki Y. Effect of calcium(II) on the interaction between the subunits of troponin and tropomyosin. Biochemistry. 1973 Nov 20;12(24):4972–4980. doi: 10.1021/bi00748a024. [DOI] [PubMed] [Google Scholar]
- Weber A., Murray J. M. Molecular control mechanisms in muscle contraction. Physiol Rev. 1973 Jul;53(3):612–673. doi: 10.1152/physrev.1973.53.3.612. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Wilkinson J. M., Perry S. V., Cole H. A., Trayer I. P. The regulatory proteins of the myofibril. Separation and biological activity of the components of inhibitory-factor preparations. Biochem J. 1972 Mar;127(1):215–228. doi: 10.1042/bj1270215. [DOI] [PMC free article] [PubMed] [Google Scholar]