Abstract
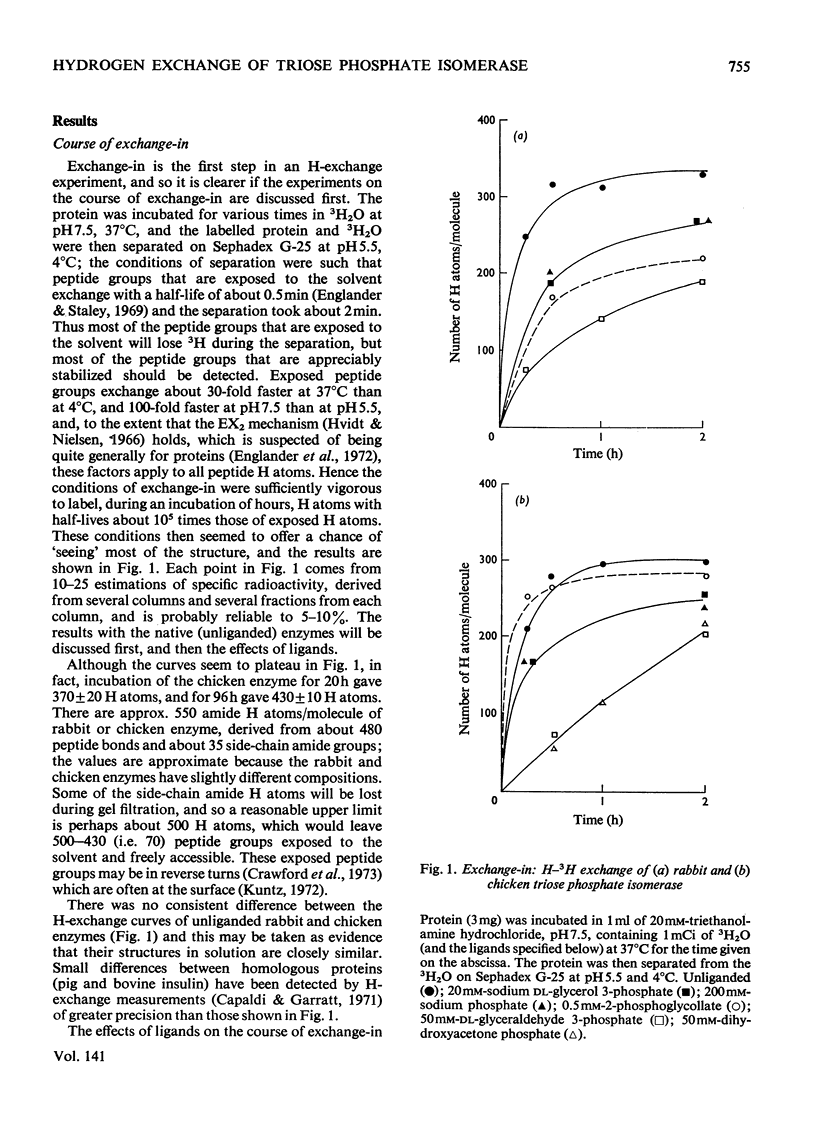
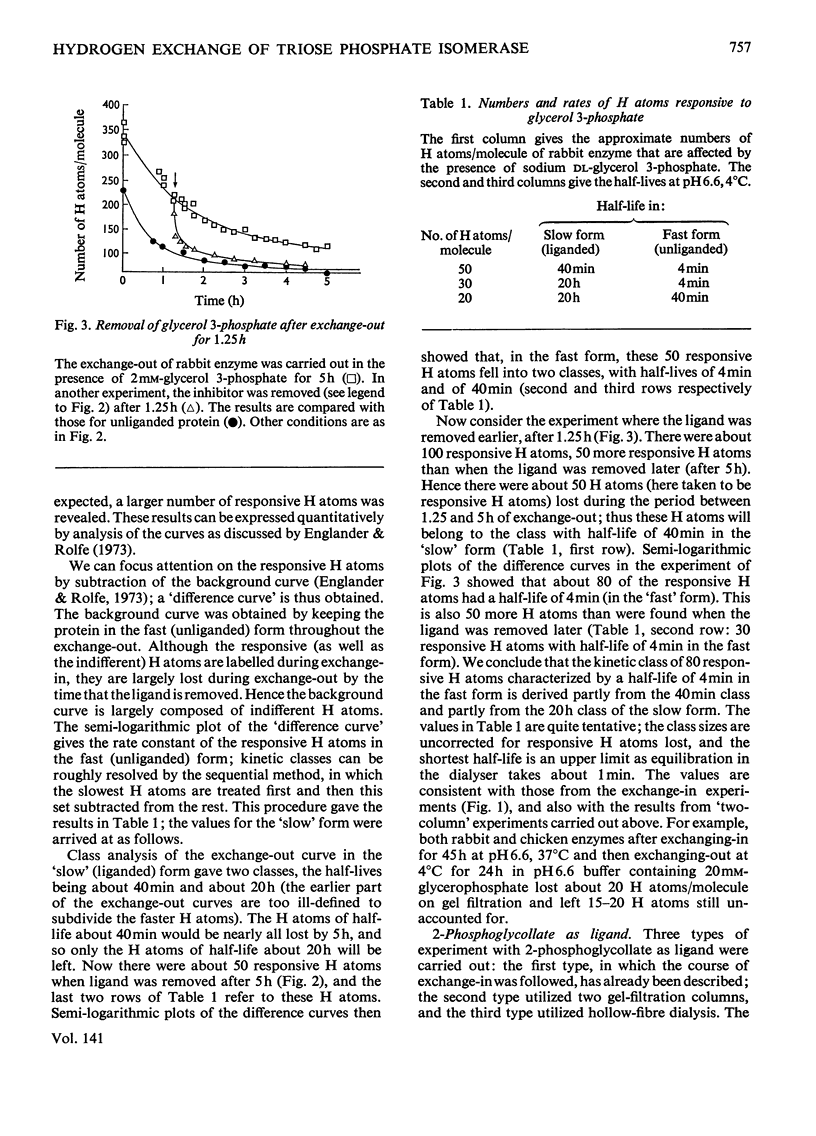
The 3H–H exchange of chicken muscle and rabbit muscle triose phosphate isomerases was studied. Their behaviour was mostly very similar. `Exchange-in' (acquisition of radioactivity when protein was incubated in 3H2O) was measured at 37°C and at pH7.5, and the rates of exchange of the native and liganded enzymes were compared. Inhibitors and substrates retarded exchange, substrates showing the most marked effect; structural rearrangements in the enzyme may thus play some part in catalysis. The inhibitor phosphoglycollate affected the rabbit enzyme, but had little or no effect on the chicken enzyme. `Exchange-out' (loss of radioactivity from protein previously labelled by incubation in 3H2O) was measured by hollow-fibre dialysis. When ligand was removed during the course of dialysis (by replacing buffer that contained ligand with buffer that lacked ligand) there was a prompt decrease in the number of labelled H atoms of the protein. Analysis of the curves provides some information about the number and half-lives of the responsive H atoms. Ligands decrease the motility of the protein and affect about one-fifth of the chain. Low concentrations of glycerol 3-phosphate have an effect that is greater than expected.
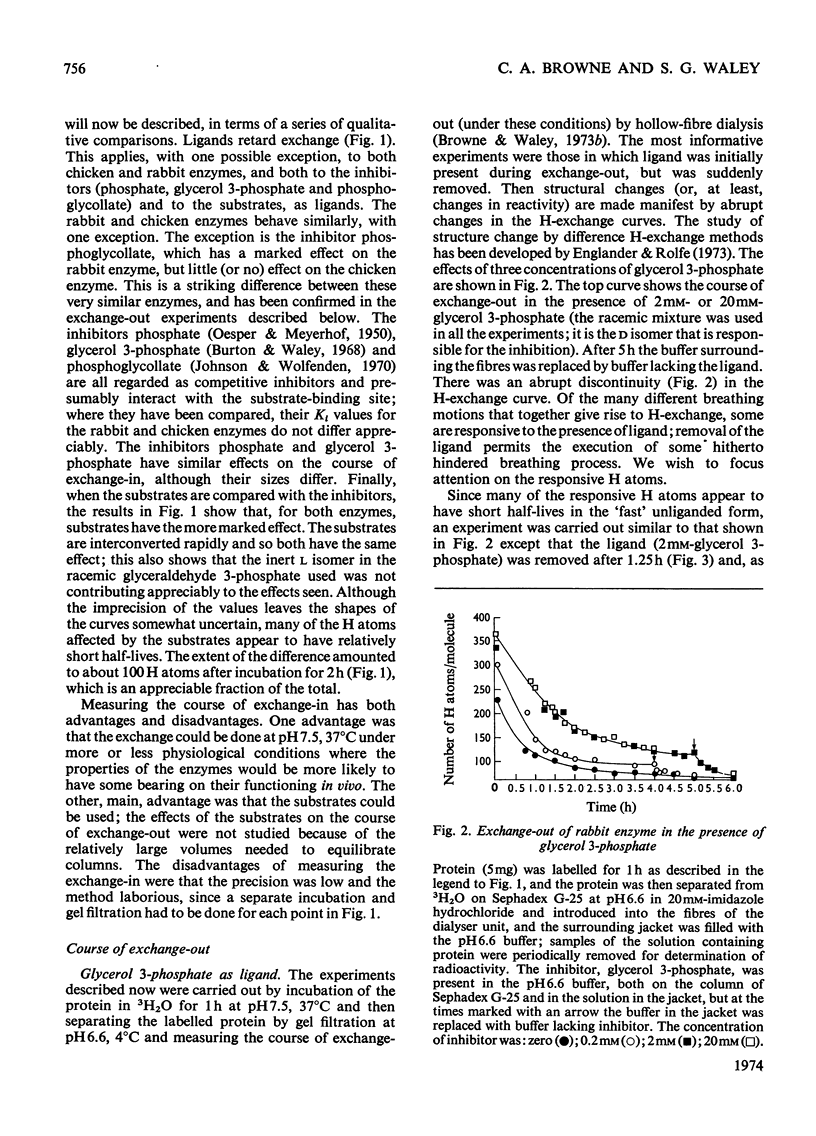
Full text
PDF

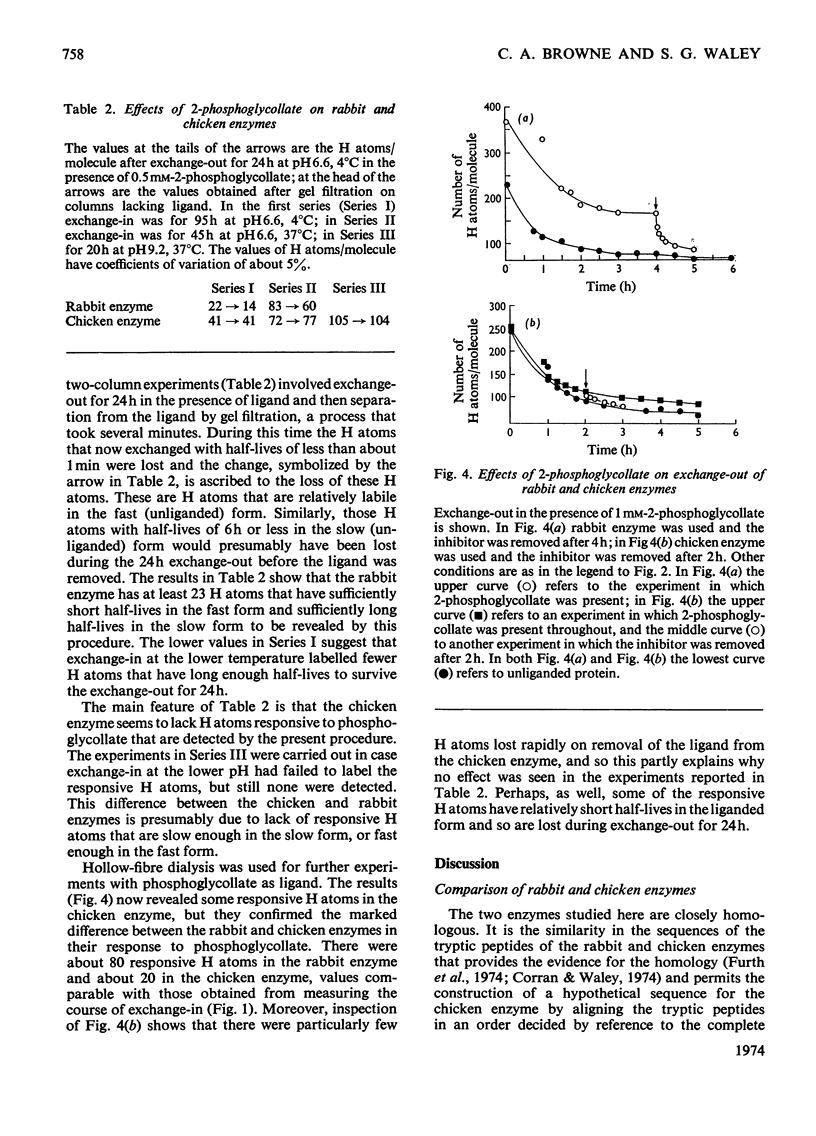


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banner D. W., Bloomer A. C., Petsko G. A., Phillips D. C., Pogson C. I. Crystallographic studies of chicken triose phosphate isomerase. Cold Spring Harb Symp Quant Biol. 1972;36:151–155. doi: 10.1101/sqb.1972.036.01.021. [DOI] [PubMed] [Google Scholar]
- Browne C. A., Waley S. G. The use of hollow-fiber dialysis in tritium-hydrogen exchange. Anal Biochem. 1973 Nov;56(1):289–291. doi: 10.1016/0003-2697(73)90191-7. [DOI] [PubMed] [Google Scholar]
- Burton P. M., Waley S. G. Kinetics of triose phosphate isomerase. Biochim Biophys Acta. 1968 Mar 25;151(3):714–715. doi: 10.1016/0005-2744(68)90028-4. [DOI] [PubMed] [Google Scholar]
- Capaldi R. A., Garratt C. J. An analysis of a structural difference between beef and pork insulin detected by differences in the exchange rates of amide hydrogens as measured by infrared spectroscopy. Eur J Biochem. 1971 Dec 10;23(3):551–556. doi: 10.1111/j.1432-1033.1971.tb01653.x. [DOI] [PubMed] [Google Scholar]
- Corran P. H., Waley S. G. The amino acid sequence of rabbit muscle triose phosphate isomerase. FEBS Lett. 1973 Feb 15;30(1):97–99. doi: 10.1016/0014-5793(73)80627-1. [DOI] [PubMed] [Google Scholar]
- Corran P. H., Waley S. G. The tryptic peptides of rabbit muscle triose phosphate isomerase. Biochem J. 1974 Apr;139(1):1–10. doi: 10.1042/bj1390001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford J. L., Lipscomb W. N., Schellman C. G. The reverse turn as a polypeptide conformation in globular proteins. Proc Natl Acad Sci U S A. 1973 Feb;70(2):538–542. doi: 10.1073/pnas.70.2.538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DIXON M. The determination of enzyme inhibitor constants. Biochem J. 1953 Aug;55(1):170–171. doi: 10.1042/bj0550170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ENGLANDER S. W. A HYDROGEN EXCHANGE METHOD USING TRITIUM AND SEPHADEX: ITS APPLICATION TO RIBONUCLEASE. Biochemistry. 1963 Jul-Aug;2:798–807. doi: 10.1021/bi00904a030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Englander S. W., Downer N. W., Teitelbaum H. Hydrogen exchange. Annu Rev Biochem. 1972;41:903–924. doi: 10.1146/annurev.bi.41.070172.004351. [DOI] [PubMed] [Google Scholar]
- Englander S. W., Rolfe A. Hydrogen exchange studies of respiratory proteins. 3. Structural and free energy changes in hemoglobin by use of a difference method. J Biol Chem. 1973 Jul 10;248(13):4852–4861. [PubMed] [Google Scholar]
- Englander S. W., Staley R. Measurement of the free and the H-bonded amides of myoglobin. J Mol Biol. 1969 Oct 28;45(2):277–295. doi: 10.1016/0022-2836(69)90105-3. [DOI] [PubMed] [Google Scholar]
- Furth A. J., Milman J. D., Priddle J. D., Offord R. E. Studies on the subunit structure and amino acid sequence of trisoe phosphate isomerase from chicken breast muscle. Biochem J. 1974 Apr;139(1):11–22. doi: 10.1042/bj1390011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hvidt A., Nielsen S. O. Hydrogen exchange in proteins. Adv Protein Chem. 1966;21:287–386. doi: 10.1016/s0065-3233(08)60129-1. [DOI] [PubMed] [Google Scholar]
- Johnson L. N., Wolfenden R. Changes in absorption spectrum and crystal structure of triose phosphate isomerase brought about by 2-phosphoglycollate, a potential transition state analogue. J Mol Biol. 1970 Jan 14;47(1):93–100. doi: 10.1016/0022-2836(70)90404-3. [DOI] [PubMed] [Google Scholar]
- Knowles J. R., Leadlay P. F., Maister S. G. Triosephosphate isomerase: isotope studies on the mechanistic pathway. Cold Spring Harb Symp Quant Biol. 1972;36:157–164. doi: 10.1101/sqb.1972.036.01.022. [DOI] [PubMed] [Google Scholar]
- Kolb E., Harris J. I., Bridgen J. Triose phosphate isomerase from the coelacanth. An approach to the rapid determination of an amino acid sequence with small amounts of material. Biochem J. 1974 Feb;137(2):185–197. doi: 10.1042/bj1370185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuntz I. D. Protein folding. J Am Chem Soc. 1972 May 31;94(11):4009–4012. doi: 10.1021/ja00766a060. [DOI] [PubMed] [Google Scholar]
- Liberti P. A., Stylos W. A., Maurer P. H. Conformational change(s) induced in sheep calcium-dependent antibody upon interaction with homologous polypeptide antigen. I. Hydrogen-exchange studies of immunoglobulin G and (Fab') 2 fragment. Biochemistry. 1972 Aug 29;11(18):3312–3320. doi: 10.1021/bi00768a002. [DOI] [PubMed] [Google Scholar]
- Miller J. C., Waley S. G. Amino acid sequences around the cysteine residues of rabbit muscle triose phosphate isomerase. Biochem J. 1971 Apr;122(2):209–218. doi: 10.1042/bj1220209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J. C., Waley S. G. The active centre of rabbit muscle triose phosphate isomerase. The site that is labelled by glycidol phosphate. Biochem J. 1971 Jun;123(2):163–170. doi: 10.1042/bj1230163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OESPER P., MEYERHOF O. The determination of triose phosphate isomerase. Arch Biochem. 1950 Jun;27(1):223–233. [PubMed] [Google Scholar]
- Plaut B., Knowles J. R. pH-dependence of the triose phosphate isomerase reaction. Biochem J. 1972 Sep;129(2):311–320. doi: 10.1042/bj1290311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putman S. J., Coulson A. F., Farley I. R., Riddleston B., Knowles J. R. Specificity and kinetics of triose phosphate isomerase from chicken muscle. Biochem J. 1972 Sep;129(2):301–310. doi: 10.1042/bj1290301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trentham D. R., McMurray C. H., Pogson C. I. The active chemical state of D-glyceraldehyde 3-phosphate in its reactions with D-glyceraldehyde 3-phosphate dehydrogenase, aldolase and triose phosphate isomerase. Biochem J. 1969 Aug;114(1):19–24. doi: 10.1042/bj1140019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waley S. G. Refolding of triose phosphate isomerase. Biochem J. 1973 Sep;135(1):165–172. doi: 10.1042/bj1350165. [DOI] [PMC free article] [PubMed] [Google Scholar]


