Abstract
Background
Postoperative delirium (POD) is common postoperative complications in non-cardiac surgery. While delirium prophylaxis has not yielded unequivocal support. The clinical effects of glucocorticoids on POD remains unclear.
Objective
To evaluate the effects of glucocorticoids on postoperative delirium (POD) in patients undergoing non-cardiac surgery.
Design
Systematic review with meta-analysis.
Methods
In strict accordance with the PRISMA statement, a systematic literature search was undertaken across PubMed, EMBASE, Web of Science and Cochrane Library databases in May 2023. We updated the search results on June 28, 2024. We used the Grading of the Recommendation Assessment, Development, and Evaluation (GRADE) system to evaluate the quality of evidence.
Results
This meta-analysis included twelve randomized controlled trials involving 1044 participants undergoing non-cardiac surgery. Compared with the control group, glucocorticoids significantly reduced the incidence of POD in patients undergoing non-cardiac surgery (RR:0.50, 95%CI:0.41 to 0.60, P < 0.00001, I2 = 26 %, GRADE = high). Meanwhile, glucocorticoids was associated with reducing the severity of POD (RR: −0.67, 95%CI: −1.10 to −0.23, P = 0.003, I2 = 89 %, GRADE = low). However, there were no significant differences with regards to patients receiving antipsychotic drug (RR: 0.91, 95%CI:0.43 to 1.92, P = 0.80, I2 = 0 %, GRADE = moderate), length of hospital stay (RR: −0.52, 95%CI: −1.41 to 0.36, P = 0.24, I2 = 0 %, GRADE = moderate), 30-day postoperative mortality (RR: 0.70, 95%CI:0.23 to 2.15, P = 0.54, I2 = 0 %, GRADE = low) and postoperative infection (RR: 0.87 95%CI: 0.58 to 1.30, P = 0.50, I2 = 33 %, GRADE = moderate).
Conclusions
This systematic review and meta-analysis suggests that glucocorticoids reduce the incidence of POD among adults and children undergoing non-cardiac surgery and mitigate the severity of POD in adults, which indicates that glucocorticoids exhibit preventive or therapeutic effects on POD.
Registration
CRD42023426836 (PROSPERO).
Keywords: Glucocorticoids, Postoperative, Delirium, Meta-analysis, Non-cardiac surgery
Highlights
-
•
Glucocorticoids reduce the incidence of POD among adults and children undergoing non-cardiac surgery.
-
•
Glucocorticoids may attenuate the severity of POD in adults after non-cardiac surgery.
-
•
Glucocorticoids exhibit preventive or therapeutic effects on POD, which needs to be validated with larger samples.
1. Background
Postoperative delirium (POD) is an acute neurocognitive disorder that arises after surgery [1]. The cardinal features of POD include impaired attention, consciousness, and cognition. The onset of POD is usually within the first 24 h following surgery [2], and the duration ranges from several hours to a few days, with fluctuation in severity [3]. Emergency delirium (ED) is considered an acute neurological complication during recovery from anesthesia. ED may be characterized by disorientation, hallucinations, panic, depressive mood, and hyperactive physical behavior or hypoactive signs [4,5]. Postoperative delirium involves ED; ED represents the early onset of postoperative delirium [[6], [7], [8]]. ED in the PACU is a strong predictor of postoperative delirium [9]. POD has been linked to impaired postoperative recovery, prolonged length of hospital stay, escalated medical expenses, long-term cognitive decline, and heightened mortality risk [10,11]. POD is one of the most common postoperative complications in non-cardiac surgery, with an incidence of 12 %–51 % [12,13]. In this review, the terms POD and ED are used interchangeably, which differs from the approach taken in previous studies [9,14].
Currently, the efficacy of pharmacological prophylaxis for delirium remains ambiguous [15]. A systematic review indicates a lack of evidence substantiating the utilization of haloperidol or second-generation antipsychotics for delirium prophylaxis [16]. Dexmedetomidine also has some therapeutic effect on POD in clinical practice [17], but this comes at the expense of an increased risk of bradycardia and hypotension [18,19]. Thus, an alternative approach is necessitated.
The pathogenesis underlying delirium remains elusive. Neuroinflammation, which can be elicited by physiological stress, anesthetic agents, neurotransmitter imbalances, and other factors, represents a putative etiological pathway. This neuroinflammatory process is primarily incited by surgical trauma and infection, which then elicit a spectrum of immune responses. These responses induce neuronal damage, thereby precipitating delirium [20,21]. Glucocorticoids, renowned for their immunosuppressive properties, play a pivotal role in the therapy and prophylaxis of immune-mediated disorders [13]. Due to concerns over potential confounders that could alter the effects of glucocorticoids on POD [22], cardiac surgeries were excluded. Accordingly, we performed a systematic review and meta-analysis to address the hypothesis that glucocorticoids decrease the incidence and severity of POD in patients undergoing non-cardiac surgeries.
2. Methods
This meta-analysis was reported in strict accordance with the PRISMA [23] statement (Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement) and registered in PROSPERO(CRD42023426836). We used the Grading of the Recommendation Assessment, Development, and Evaluation (GRADE) system to evaluate the quality of evidence [24].
2.1. Search strategy
A systematic literature search was undertaken across PubMed, EMBASE, Web of Science and Cochrane Library databases in May 2023. The language was restricted to English. We updated the search results on June 28, 2024. Search terms included “delirium”, “glucocorticoids”, “Methylprednisolone”, “Dexamethasone”, “Prednisone”, “Hydrocortisone”, “Betamethasone”, and “Beclomethasone”. According to the search strategy, both Medical Subject Headings and Entry terms were used. In addition, the reference lists from retrieved articles were reviewed to identify potentially eligible trials.
2.2. Eligibility criteria
We included studies with the following criteria: (1) randomized controlled trials of elective non-cardiac surgery; (2) Perioperative administration of glucocorticoids encompassing preoperative, intraoperative, and postoperative infusion to safeguard against POD relative to placebo or untreated controls, irrespective of dosage regimen utilized; (3) One of the incidence or severity of POD as a primary or secondary outcome; (4) The language was restricted to English.
2.3. Exclusion criteria
Non-human studies, studies without available data can be extracted, studies devoid of accessible full text or lacking validated delirium assessment instrumentations to assess the incidence of POD.
2.4. Study selection
Two authors (JL and DW) independently screened titles and abstracts to find out relevant studies. If the title or abstract of the study was considered eligible, the full-text was retrieved. If there was a discrepancy between the two authors (JL and DW), it was resolved by discussion with another senior researcher(LPH).
2.5. Data extraction
With a pre-designed table, two authors (JL and DW) independently carried out the data extraction. The disputes were resolved by the third author (LPH). The demographics and outcome data were extracted. The demographics of included studies embraced author and region, sample size, mean age, type of surgery, duration of surgery, type of anesthesia, intervention, type of glucocorticoid and dosage, control group, POD assessment tool, primary outcome, secondary outcome. Results reported in median (interquartile range) or median (interquartile range [range]) were calculated to mean (standard deviation) using the methods of Luo and Wan et al. [[25], [26], [27]].
The following outcomes were applied for comparison: the incidence and severity of POD, patients receiving antipsychotics drug, length of hospital stay, 30-day postoperative mortality, and postoperative infection. When the evaluation time-points are different, the results with the longest interval are included.
2.6. Risk of bias and quality assessment
Two authors (JL and DW) evaluated the risk of bias of included RCTs using the Cochrane Handbook for Systematic Reviewers (version 5.1.0) RCT risk of bias assessment instruments. Evaluation indicators include: (1) sequence generation (selection bias); (2) allocation concealment (selection bias); (3) blinding of patients and personnel (performance bias); (4) blinding of outcome assessors (detection bias); (5) incomplete outcome data (attrition bias); (6) elective reporting (reporting bias); (7) other bias. Each indicator encompasses three stratified levels: low risk, unclear and high risk. Disagreements were resolved by consulting a third reviewer (LPH).
2.7. Statistical analysis and data synthesis
Statistical analyses were performed with Review Manager 5.4.1 (Cochrane Collaboration, Oxford, UK) and Stata 16.0 (Stata Corp LP, College Station, Texas). Binary outcomes were calculated as relative risk (RR). Effect sizes were calculated as weighted mean difference (WMD) and 95 % confidence interval (95%CI) when the assessment tools for continuous outcomes were the same. In other cases, when different delirium assessment tools are employed, the standard mean difference (SMD) and 95 % confidence intervals (95 % CI) are utilized. Statistical heterogeneity among studies was evaluated using Cochran's Q test and Higgins I2 statistics. If I2 > 50 % or p < 0.05 (indicating significant heterogeneity among studies), the data were combined using a random effects model. On the contrary, a fixed effects model was conducted. Sensitivity analysis was also conducted to evaluate the effect of the individual study data. Publication bias was evaluated via funnel plot analysis, with significant bias defined as obvious asymmetry. When I2 > 50 % or p < 0.05 (indicating significant heterogeneity among studies), and subgroup analysis was performed to find the resource of heterogeneity. We planned to perform subgroup analysis by age and type of glucocorticoid.
3. Results
3.1. Study selection
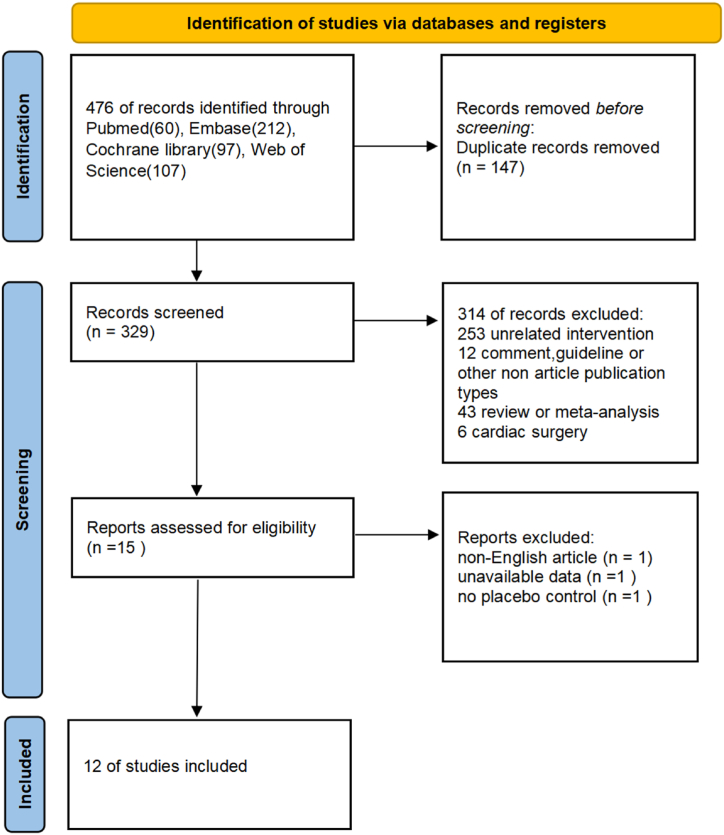
According to the search strategy, a total of 476 potentially eligible studies were discerned. Among them, 147 studies were removed due to duplication. Subsequent to screening per titles and abstracts, 329 studies failing to fulfill eligibility criteria were excluded. Ultimately, 15 full-text articles were assessed for eligibility. In total, 12 RCTs could be included in our analysis. The flow of study selection was shown in Fig. 1.
Fig. 1.
Study flowchart showing results of selection.
3.2. Study characteristics
The detailed characteristics of these eligible studies are presented in Table 1. Seven of the included studies involved adults [[28], [29], [30], [31], [32], [33], [34]], the others involved children [[35], [36], [37], [38], [39]]. Five of the included studies were hip fracture surgery [28,[31], [32], [33], [34]], two trials were adenotonsillectomy [38,39], the other five studies were gastrointestinal surgery [29], urologic surgery [30], upper gastrointestinal endoscopy (UGIE) [35], dental surgery and cleft palate repair surgery respectively [36,37]. Among the included studies, barring two employing intrathecal delivery [32,33], intravenous administration constituted the route of drug infusion. The outcome measures and assessment instruments were explicitly delineated across all studies.
Table 1.
Characteristics of the included studies.
| Study ID | Region | No. of participants | Age(y) | Type of surgery | Surgery duration | Type of anesthesia | Intervention | Placebo | Delirium assessment | Primary outcome | Secondary outcomes |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Clemmesen 2018 | Denmark | MET:59 PLA:58 | MET: 79 ± 8 PLA: 81 ± 9 | Hip fracture surgery | MET: 69 (51–85) PLA: 74 (49–83) | GA/EA/SA + EA | 125 mg, i.v. | Not reported | CAM- S | Severity of delirium | Incidence of delirium, CAS, VRS, Antipsychotic drug administered, Infection, Length of stay, Completed/partially completed physiotherapy, Pain on ambulation, 30-day postoperative mortality, 90-day postoperative mortality |
| Xiang 2022 | China | MET:84 PLA:84 | MET: 71 (68–74) PLA: 70 (68–73) | Gastrointestinal surgery | MET: 177 (137.5–213.8) PLA: 161 (128.3–210.0) | GA | 2 mg/kg, i.v. | NS | CAM-S | Incidence of delirium | Cumulative CAM-S score, Patients received haloperidol, Exhausttime, Infection, Anastomotic leakage, NRS, Length of stay, 30-day mortality |
| Cho 2022 | Korea | DEX: 45 PLA: 45 | DEX: 59.6 ± 9.0 PLA: 54.4 ± 15.4 | Urologic surgery | DEX: 55.0 (27.5–90.0) PLA: 40.0 (27.5–60.0) | GA | 10 mg. i.v. | NS | RSAS | Incidence and severity of CRBD | Incidence and severity of delirium, NRS pain score |
| Kluger 2021 | New Zealand | DEX: 40 PLA: 39 | DEX: 81.4 ± 7.2 PLA: 81.4 ± 8.9 | Hip fracture surgery | DEX: 165 ± 39 PLA: 150 ± 36 | GA/SA/SA + GA | 20 mg, i.v. | NS | 4AT + MDAS | Incidence and severity of delirium | Pain at rest, Pain on movement, Length of stay, Mortality 30 days, Mortality 6 months, |
| Moheimani 2019 | Iran | DEX: 49 PLA: 49 | DEX: 7.8 ± 2.8 PLA: 7.2 ± 3 | UGIE | Not reported | GA | 0.1 mg/kg, i.v. | NS | PAED | Incidence of PONV | The incidence of bronchospasm or laryngospasm, Emergence delirium score, Modified Aldrete score, Patient recovery time |
| Shama 2023 | Egypt | DEX: 25 PLA: 25 | DEX: 8.52 ± 1.50 PLA: 8.80 ± 1.35 | Dental surgery | DEX: 101.60 ± 32.68 PLA: 106.12 ± 29.98 | GA | 0.15 mg/kg, i.v. | NS | PAED | Incidence of PONV | Incidence of delirium, PAED score, Number and percentage of patients requiring rescue antiemetic, Postoperative pain, Postsurgical complications |
| Elsonbaty 2016 | Egypt | DEX: 30 PLA: 30 | DEX:3.7 ± 1.36 PLA:3.6 ± 1.16 | Cleft palate repair surgery | Not reported | GA | 0.15 mg/kg, i.v. | Blank control | Watcha score | Incidence of delirium | Blood glucose level, Incidence of PONV |
| Sakic 2015 | Croatia | DEX: 17 PLA: 11 | DEX: 83 (73–95) PLA: 78 (54–91) | Hip fracture surgery | DEX: 114.54 (65.15–170) PLA: 108.30 (63–175) | SA | 8 mg, i.t. | Blank control | CAM | Incidence of delirium, Plasma cortisol level | Severity of pain, Blood glucose level, Recovery |
| Sajedi 2014 | Iran | DEX: 32 PLA: 32 | DEX: 4.56 ± 1.2 PLA: 4.71 ± 1.3 | Adenotonsillectomy | DEX: 41 ± 7.8 PLA: 42.18 ± 6.4 | GA | 0.2 mg/kg, i.v. | NS | Richmond agitation sedation score | Incidence of delirium | Incidence of pain and complications, Recovery time, Duration of agitation, Time to agitation appearance, Meperidine consumption, Meperidine consumption, Midazolam consumption, Nurse satisfaction score, Time to extubation, Anesthesia time, Surgery time |
| Khalili 2012 | Iran | DEX: 35 PLA: 35 | DEX: 4.71 ± 1.33 PLA: 4.66 ± 1.16 | Adenotonsillectomy | DEX: 39.86 ± 11.5 PLA: 39.12 ± 11.3 | GA | 0.2 mg/kg, i.v. | NS | 5 point rating scale | Incidence of delirium | Incidence of pain, Mean agitation and pain scores |
| Sakic2023 | Croatia | DEX: 30 PLA: 30 | DEX: 81.63 ± 6.94 PLA: 79.69 ± 10.17 | Hip fracture surgery | Not reported | SA | 8 mg, i.t. | Blank control | CAM | Plasma cortisol level, Incidence of cognitive disturbances | Duration of analgesia, Length of hospital stay, Postoperative pain intensity at first hour postop, as well as the third, fifth and tenth days after surgery |
| Huang 2023 | China | DEX: 80 PLA: 80 | DEX: 84.5 (79.0–89.0) PLA: 85.0 (79.8–90.2) | Hip fracture surgery | DEX: 76.0 (62.8–90.5) PLA: 74.0 (60.0–93.5) | GA + SA | 10 mg, i.v. | NS | Nu-DESC + MDAS | Incidence and severity of delirium | Infection, Hyperglycemia, Maximum glucose in the frst 3 days postoperatively |
Date presented as mean ± SD or median (interquartile range); CAM = Confusion Assessment Method; CAM-S = Confusion Assessment Method—Severity; CAS = Cumulated Ambulation Score; DEX = dexamethasone; EA = epidural anesthesia; GA = general anesthesia; i.v. = intravenous injection; i.t. = intrathecal injection; MET = methylprednisolone; MDAS = Memorial Delirium Assessment Scale; Nu-DESC = Nursing Delirium Screening Scale; NS = normal saline; NRS = numerical rating scale; PLA = placebo; PONV = postoperative nausea and vomiting; PAED = Pediatric Anesthesia Emergence Delirium; RSAS = Riker Sedation-Agitation Scale; RASS = Richmond Agitation Sedation Scale; SA = spinal anesthesia; UGIE = upper gastrointestinal endoscopy; VRS = verbal rating scale; 4AT = arousal, attention, abbreviated Mental Test.
3.3. Risk of bias in the included studies
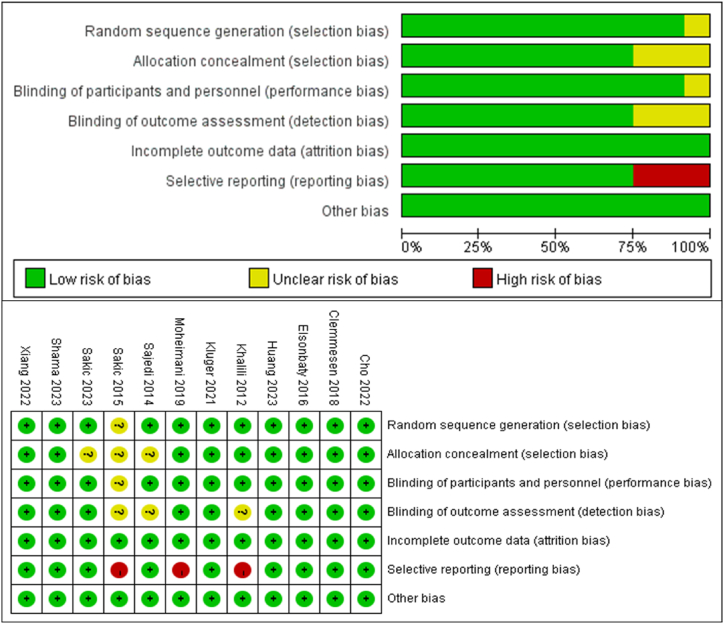
Risk of bias and quality assessment was conducted according to Cochrane Handbook for Systematic Reviewers and the result was presented in Fig. 2. According to the primary outcome, three studies had a high risk of bias because of reporting bias [32,35,39].
Fig. 2.
Risk of bias assessment for each included study per the Cochrane risk of bias framework.
3.4. Primary outcomes
3.4.1. Incidence of POD
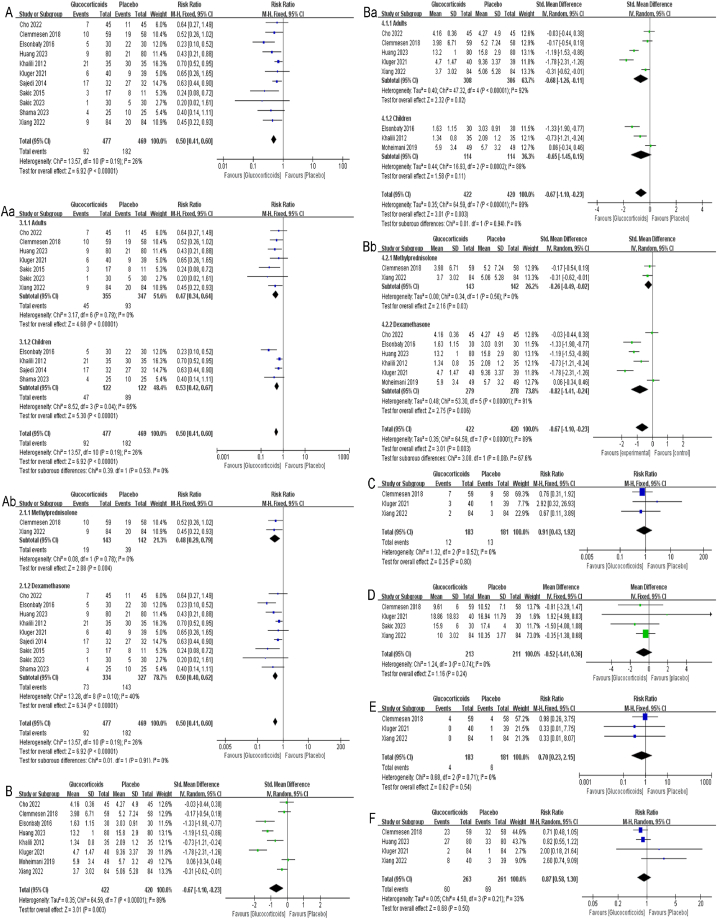
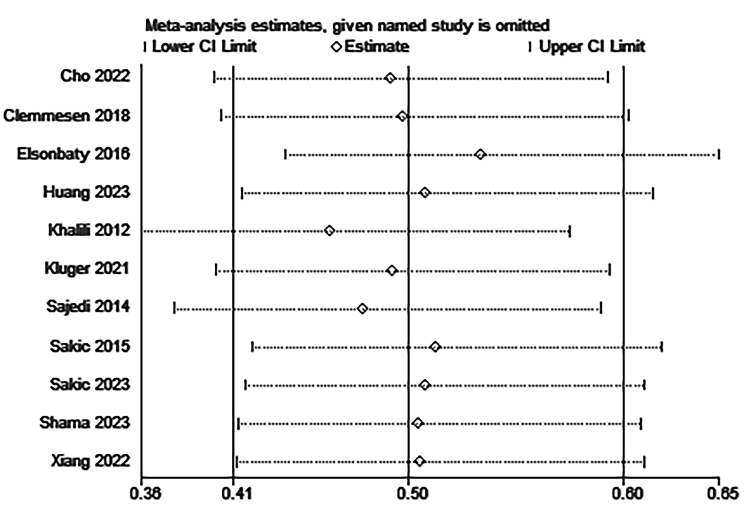
Eleven studies with a total of 946 patients reported the incidence of POD, in which data were reported as the number of participants [[28], [29], [30], [31], [32], [33], [34],[36], [37], [38], [39]]. The delirium evaluation time-points ranged from 10 min to 5 days across the eleven studies. Hence, the final assessable dataset was utilized for meta-analysis. The results showed that glucocorticoids significantly reduced the incidence of POD among adults and children undergoing non-cardiac surgery contrast with control group (RR:0.50, 95%CI:0.41 to 0.60, P < 0.00001, I2 = 26 %) (Fig. 3 A). Sensitivity analysis of the incidence of POD was performed by excluding each study individually and the results were found to be stable (Fig. Supplementary 1). Per the predefined stratification scheme, this finding was consistent in another subgroup analysis between adults (RR:0.47, 95%CI:0.34 to 0.64, P < 0.00001, I2 = 0 %) and children (RR:0.53, 95%CI:0.42 to 0.67, P < 0.00001, I2 = 65 %) (Fig. 3 Aa). Additionally, subgroup analysis stratified by the type of glucocorticoids demonstrated no significant differences (P = 0.91) between methylprednisolone (RR:0.48, 95%CI:0.29 to 0.79, P = 0.004, I2 = 0 %) and dexamethasone (RR:0.50, 95%CI:0.40 to 0.62, P < 0.00001, I2 = 40 %) (Fig. 3 Ab).
Fig. 3.
A: Forest plot of the incidence of POD; Aa, Ab: Subgroup analysis of the incidence of POD stratified by age classification and the type of glucocorticoids; B: Forest plot of the severity of POD; Ba, Bb: Subgroup analysis of the severity of POD stratified by age classification and the type of glucocorticoids; C: Forest plot of patients received antipsychotic drug; D: Forest plot of length of hospital stay; E: Forest plot of 30-day postoperative mortality; F: Forest plot of postoperative infection.
3.4.2. Severity of POD
Eight studies reported the severity of POD [[28], [29], [30], [31],34,35,37,39], in which the data were reported as cumulative scores on the respective delirium assessment tools. Similarly, the final assessable dataset was utilized for meta-analysis. In the studies conducted by Clemmesen, Xiang, Kluger et al. [28,29,31], data were reported as median (interquartile range) or median (interquartile range [range]). The approaches delineated by Luo and Wan et al. [[25], [26], [27]] were implemented to transform these data into mean (standard deviation) based on a predefined scheme. Meta-analysis results demonstrated that glucocorticoids conferring significant mitigation of delirium severity (RR: −0.67, 95%CI: −1.10 to −0.23, P = 0.003, I2 = 89 %) (Fig. 3 B). However, the finding warrant judicious interpretation given the high heterogeneity observed. Despite we conducted two subgroup analyses, the source of heterogeneity remained unidentified (Fig. 3 Ba, Fig. 3 Bb). Additionally, the subgroup analysis based on age classification (RR: −0.65, 95%CI: −1.45 to 0.15, P = 0.11, I2 = 88 %) did not demonstrate statistically significant effects of glucocorticoids in mitigating delirium severity of children.
3.5. Secondary outcomes
The patients received antipsychotic drug (RR: 0.91, 95%CI: 0.43 to 1.92, P = 0.80, I2 = 0 %) (Fig. 3C) and 0-day postoperative mortality (RR: 0.70, 95%CI: 0.23 to 2.15, P = 0.54, I2 = 0 %) (Fig. 3E) were reported in same three studies [28,29,31], results showed there was no significant difference between the glucocorticoids group and control group. In addition, length of hospital stay following surgery [28,29,31,33] (RR: −0.52, 95%CI: −1.41 to 0.36, P = 0.24, I2 = 0 %) (Fig. 3D) and postoperative infection [28,29,31,34] (RR: 0.87, 95%CI: 0.58 to 1.30, P = 0.50, I2 = 33 %) (Fig. 3F) were documented in four studies, again not showing statistically significant differences with meta-analyses results. We should interpret the conclusion of postoperative infection carefully because of the heterogeneity. However, additional meta-analyses were precluded by the paucity of data.
3.6. Publication bias
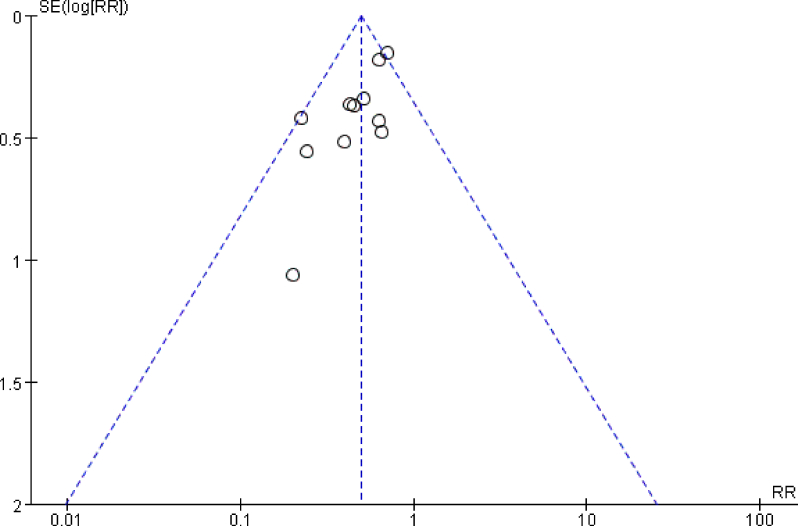
There was no significant publication bias examined by ocular-estimation of funnel plot (Fig. Supplementary 2) for the effects of glucocorticoids administration on POD.
3.7. Level of certainty for outcomes (GRADE)
Employing the GRADE system, the certainty of evidence for main outcomes was appraised, exhibiting level of certainty as delineated in Table 2.
Table 2.
GRADE evidence for outcomes.
| Outcomes | No of studies | No of patients | Quality assessment | Quality | ||||
|---|---|---|---|---|---|---|---|---|
| Risk of bias | Inconsistency | Indirectness | Imprecision | Publication bias | ||||
| Incidence of delirium | 11 | 946 | Not serious | Not serious | Not serious | Not serious | Not serious | High |
| Cumulative score | 8 | 842 | Not serious | Seriousa | Not serious | Seriousc | Not serious | Low |
| Patients received antipsychotic drug | 3 | 364 | Not serious | Not serious | Not serious | Seriousd | Not serious | Moderate |
| Length of hospital stay | 4 | 424 | Not serious | Not serious | Not serious | Seriousc | Not serious | Moderate |
| 30-day postoperative mortality | 3 | 364 | Not serious | Not serious | Seriousb | Seriousd,e | Not serious | Low |
| Infection | 4 | 524 | Not serious | Seriousa | Not serious | Serious | Not serious | Moderate |
Inconsistency due to significant statistical heterogeneity.
Potential confounding by the underlying pathology and surgical modality cannot be excluded.
We used the median (interquartile range) or median (interquartile range [range]) to approach the means (SD), which might decrease confidence in the estimate and the 95 % CI.
The quality was rated for imprecision due to total sample size is less than 400.
The quality was rated for imprecision due to the wide range of 95 % confidence intervals.
4. Discussion
This systematic review and meta-analysis suggested that glucocorticoids reduce the incidence of POD among both adults and children after non-cardiac surgery, a finding consistent across two subgroup analyses. Moreover, glucocorticoid infusion was associated with a reduction in the severity after the onset of delirium. However, no significant difference was observed between groups in patients receiving antipsychotic drugs, in the length of hospital stay, in 30-day postoperative mortality, or in postoperative infection rates. According to the GRADE framework, the certainty of the evidence varied from low to high.
Although the pathogenesis of delirium is unknown, studies have shown that patients' chronological age, preoperative cognitive function, anesthetic dosage, neuro-inflammation, cardiopulmonary bypass, temperature management, postoperative pain, duration of surgery, and other factors are associated with the incidence of POD [22,[40], [41], [42], [43], [44]]. Neuroinflammation is pivotal in the initiation of delirium, and systemic injury can lead to elevated levels of the pro-inflammatory cytokine IL-1β within regions previously affected in the central nervous system [20,21]. Preoperative glucocorticoids have been used across various surgical contexts to mitigate the harmful effects of inflammation caused by surgical trauma and anesthetic exposure [45]. Multiple studies have demonstrated that preoperative glucocorticoid treatment reduces peripheral inflammatory markers in hepatic resection [46,47]. Based on these findings, it seems plausible that glucocorticoids may offer preventative or therapeutic benefits against delirium.
There were three similar meta-analyses [[48], [49], [50]] which drew the opposite conclusion versus this review. Our meta-analysis differs from the aforementioned three studies in that they all included patients undergoing cardiac surgery while omitting pediatric patients. On the one hand, potential reasons underlying the discrepant results may relate to heightened delirium incidence following cardiac versus non-cardiac surgery, attributable to cardiopulmonary bypass, specialized anesthetic regimens, augmented drug administration, prolonged operative duration, extensive surgical insult in the former and so on, thus underestimating the effects of glucocorticoids on postoperative delirium. In a study by Hovens et al. [51], both cardiac and abdominal surgery induced changes in hippocampal BDNF signaling in rats, while increased plasma and NGAL (neutrophil gelatinase associated lipocalin) activity in the hypothalamic paraventricular nucleus and microglia activity in the hippocampus and prefrontal cortex after cardiac surgery, but not after abdominal surgery. These results suggest that cardiac surgery has more extensive and complex effects on the brain than non-cardiac surgery.
On the other hand, although advanced age constituting a salient POD risk factor [52,53], the susceptibility of delirium is not confined to elderly individuals, but contingent on the precipitating risk factors across all age groups [52]. Moreover, pediatric and adult patients exhibited a similar spectrum of delirium symptoms. The onset of delirium was rapid in both groups, and they presented with fluctuating symptoms, including attention and consciousness disorders, cognitive impairments, sleep-wake cycle disruptions, neuromotor abnormalities, and emotional disturbances. The gold standard for the diagnosis of delirium in children and adults is based on the criteria set forth in the 5th edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-5) issued by the American Psychiatric Association, which is applicable to both adult and pediatric patients [21,54]. Additionally, ED in pediatric patients represents an early stage of POD within the spectrum of delirium progression [6,7]. As such, it is not unexpected that both conditions are incorporated in the analysis when considering the development of delirium. The insufficient number of included studies may have affected the outcome of glucocorticoids on POD in patients undergoing non-cardiac surgery. Our subgroup analysis showed that perioperative glucocorticoid infusion was equally effective in controlling POD in non-adult patients (children). Nevertheless, the results require judicious interpretation given the possibility of small study bias introduced by the cumulative sample size of 244 patients across the 4 studies. Besides, statistical analysis exhibited significant heterogeneity within the children subgroup. Sequential exclusion of individual studies revealed markedly reduced heterogeneity (I2 = 0 %) upon ruling out the study of Elsonbaty et al. [37]. Potential reason includes variances in delirium assessment instruments, where the Watcha scale is a simpler tool and may confer higher sensitivity compared to the PAED scale [55], with possible preclusion of additional in-depth analyses due to insufficient incorporated data.
Based on our approach, we included studies with "incidence or severity of POD" as the endpoint. However, the studies we included were not able to make recommendations on the optimal dose of glucocorticoids to prevent delirium. Notably, while perioperative glucocorticoid administration could enhance patient outcomes by attenuating inflammation, potential adverse sequela including osteoporosis, osteonecrosis, cardiovascular disease and so on must also be considered [56]. Thus, a reliable optimal dosage remains unresolved, pending further research.
To the best of our knowledge, this is the first meta-analysis of the effects of glucocorticoids on the severity of POD. The results of meta-analysis showed that there was a statistically significant difference (P = 0.003) on reducing the severity of POD in adults undergoing non-cardiac surgery. Regrettably, the results revealed a significant degree of heterogeneity. The conducted subgroup analyses confirmed that neither age nor hormone type contributed to the observed heterogeneity. Considering the diverse patient populations, surgical procedures, and glucocorticoid dosages, clinical heterogeneity is likely the underlying reason for the observed variation. However, the scarcity of available data hinders more extensive investigations into potential sources of heterogeneity. A low quality of evidence was rated by GRADE precluding definitive recommendations. The cause of the low quality of evidence as shown in Table 2. Our subgroup analysis based on age classification suggested that perioperative administration of glucocorticoids did not reduce the severity of POD in children. Although, the children subgroup analysis is comprised merely of three studies with limited sample sizes, and the results exhibited substantial heterogeneity in the results. Furthermore, it is unreasonable to conclude that glucocorticoids are associated with an increased risk of infection [56] based on the results of the meta-analysis, as confirmed by another meta-analysis encompassing 37 studies substantiates this notion [57]. In addition, we investigated patients who received antipsychotic drug, length of hospital stay, 30-day postoperative mortality. Less data was included as secondary outcomes, which decreased the quality of evidence and required dedicated research to uncover more valuable information.
4.1. Limitations
Initially, while neuroinflammation represents a potential etiological factor in delirium episodes, insufficient data currently precludes a comprehensive analysis of the correlation between delirium incidence and inflammatory mediators like cortisol. Future research endeavors should prioritize addressing this gap in understanding. Besides, there are several potential limitations in this study that parallel those encountered in other meta-analyses of a similar nature. First, the sample sizes of all included studies were small, which generally implies a risk of small study effect bias; the quality of evidence for secondary outcomes is therefore downgraded. Second, not all endpoints correlated with perioperative glucocorticoid administration were included, with preferential inclusion of commonly documented occurrences as outcomes, potentially predisposing to omission of significant adverse sequelae and clinical endpoints. Third, time-points of delirium evaluation ranged from 10 min to 5 days postoperatively across the included studies, with variability in assessment instrumentation. Since data from the final evaluable time-point was synthesized, timing disparities could impact the observed incidence of postoperative delirium (POD). Furthermore, variability in sensitivity among assessment tools may additionally influence the measurement of the incidence of POD. Fourth, it is very difficult to perform a subgroup analysis based on the dose of glucocorticoids administration due to a few included studies had identical dosing regimens. To prevent POD after non-cardiac surgery, future trials should adopt a standardized protocol. Additionally, the included studies involved emergence agitation (EA), which was also referred to as emergence delirium (ED). The terms EA and ED have been used interchangeably in several studies [58,59]. Moreover, the same assessment tools (e.g., the Riker Sedation-Agitation Scale or the Richmond Agitation-Sedation Scale) have been used for both EA and ED.
5. Conclusion
In this meta-analysis of 12 RCTs, glucocorticoids demonstrated significant benefits in reducing the incidence of POD among adults and children after non-cardiac surgery. In addition, glucocorticoids may be associated with attenuating the severity of POD in adults after non-cardiac surgery, which needs to be validated in clinical studies with larger samples using recognized evaluation criteria.
CRediT authorship contribution statement
Zicen Li: Writing – original draft, Visualization, Software, Methodology, Investigation, Formal analysis, Data curation. Jing Lu: Supervision, Formal analysis. Di Wang: Methodology, Formal analysis. Liping Han: Writing – review & editing, Project administration, Methodology, Conceptualization.
Ethical statement
The research was conducted according to ethical standards.
Data availability statement
As this research is a meta-analysis of previous data, no new data were generated.
Funding
None to declare.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2024.e40914.
Appendix A. Supplementary data
The following are the supplementary data to this article:
figs1.
figs2.
References
- 1.Slooter A.J.C., Otte W.M., Devlin J.W., Arora R.C., Bleck T.P., Claassen J., et al. Updated nomenclature of delirium and acute encephalopathy: statement of ten Societies. Intensive Care Med. 2020;46:1020–1022. doi: 10.1007/s00134-019-05907-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chaiwat O., Chanidnuan M., Pancharoen W., Vijitmala K., Danpornprasert P., Toadithep P., et al. Postoperative delirium in critically ill surgical patients: incidence, risk factors, and predictive scores. BMC Anesthesiol. 2019;19:39. doi: 10.1186/s12871-019-0694-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Robinson T.N., Raeburn C.D., Tran Z.V., Angles E.M., Brenner L.A., Moss M. Postoperative delirium in the elderly: risk factors and outcomes. Ann. Surg. 2009;249:173–178. doi: 10.1097/SLA.0b013e31818e4776. [DOI] [PubMed] [Google Scholar]
- 4.Dahmani S., Delivet H., Hilly J. Emergence delirium in children: an update. Curr. Opin. Anaesthesiol. 2014;27:309–315. doi: 10.1097/aco.0000000000000076. [DOI] [PubMed] [Google Scholar]
- 5.Munk L., Andersen G., Møller A.M. Post-anaesthetic emergence delirium in adults: incidence, predictors and consequences. Acta Anaesthesiol. Scand. 2016;60:1059–1066. doi: 10.1111/aas.12717. [DOI] [PubMed] [Google Scholar]
- 6.Lepousé C., Lautner C.A., Liu L., Gomis P., Leon A. Emergence delirium in adults in the post-anaesthesia care unit. Br. J. Anaesth. 2006;96:747–753. doi: 10.1093/bja/ael094. [DOI] [PubMed] [Google Scholar]
- 7.Radtke F.M., Franck M., Hagemann L., Seeling M., Wernecke K.D., Spies C.D. Risk factors for inadequate emergence after anesthesia: emergence delirium and hypoactive emergence. Minerva Anestesiol. 2010;76:394–403. [PubMed] [Google Scholar]
- 8.Xará D., Silva A., Mendonça J., Abelha F. Inadequate emergence after anesthesia: emergence delirium and hypoactive emergence in the Postanesthesia Care Unit. J. Clin. Anesth. 2013;25:439–446. doi: 10.1016/j.jclinane.2013.02.011. [DOI] [PubMed] [Google Scholar]
- 9.Huang J., Qi H., Lv K., Chen X., Zhuang Y., Yang L. Emergence delirium in elderly patients as a potential predictor of subsequent postoperative delirium: a descriptive correlational study. J Perianesth Nurs. 2020;35:478–483. doi: 10.1016/j.jopan.2019.11.009. [DOI] [PubMed] [Google Scholar]
- 10.Salluh J.I., Wang H., Schneider E.B., Nagaraja N., Yenokyan G., Damluji A., et al. Outcome of delirium in critically ill patients: systematic review and meta-analysis. Bmj. 2015;350 doi: 10.1136/bmj.h2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van den Boogaard M., Schoonhoven L., Evers A.W., van der Hoeven J.G., van Achterberg T., Pickkers P. Delirium in critically ill patients: impact on long-term health-related quality of life and cognitive functioning. Crit. Care Med. 2012;40:112–118. doi: 10.1097/CCM.0b013e31822e9fc9. [DOI] [PubMed] [Google Scholar]
- 12.Schenning K.J., Deiner S.G. Postoperative delirium in the geriatric patient. Anesthesiol. Clin. 2015;33:505–516. doi: 10.1016/j.anclin.2015.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maldonado J.R. Acute brain failure: pathophysiology, diagnosis, management, and sequelae of delirium. Crit. Care Clin. 2017;33:461–519. doi: 10.1016/j.ccc.2017.03.013. [DOI] [PubMed] [Google Scholar]
- 14.Zhang Y., He S.T., Nie B., Li X.Y., Wang D.X. Emergence delirium is associated with increased postoperative delirium in elderly: a prospective observational study. J. Anesth. 2020;34:675–687. doi: 10.1007/s00540-020-02805-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marcantonio E.R. Delirium in hospitalized older adults. N. Engl. J. Med. 2017;377:1456–1466. doi: 10.1056/NEJMcp1605501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oh E.S., Needham D.M., Nikooie R., Wilson L.M., Zhang A., Robinson K.A., et al. Antipsychotics for preventing delirium in hospitalized adults: a systematic review. Ann. Intern. Med. 2019;171:474–484. doi: 10.7326/m19-1859. [DOI] [PubMed] [Google Scholar]
- 17.Nguyen V., Tiemann D., Park E., Salehi A. Alpha-2 agonists. Anesthesiol. Clin. 2017;35:233–245. doi: 10.1016/j.anclin.2017.01.009. [DOI] [PubMed] [Google Scholar]
- 18.Wu M., Liang Y., Dai Z., Wang S. Perioperative dexmedetomidine reduces delirium after cardiac surgery: a meta-analysis of randomized controlled trials. J. Clin. Anesth. 2018;50:33–42. doi: 10.1016/j.jclinane.2018.06.045. [DOI] [PubMed] [Google Scholar]
- 19.Pan H., Liu C., Ma X., Xu Y., Zhang M., Wang Y. Perioperative dexmedetomidine reduces delirium in elderly patients after non-cardiac surgery: a systematic review and meta-analysis of randomized-controlled trials. Can. J. Anaesth. 2019;66:1489–1500. doi: 10.1007/s12630-019-01440-6. [DOI] [PubMed] [Google Scholar]
- 20.Maclullich A.M., Ferguson K.J., Miller T., de Rooij S.E., Cunningham C. Unravelling the pathophysiology of delirium: a focus on the role of aberrant stress responses. J. Psychosom. Res. 2008;65:229–238. doi: 10.1016/j.jpsychores.2008.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wilson J.E., Mart M.F., Cunningham C., Shehabi Y., Girard T.D., MacLullich A.M.J., et al. Delirium. Nat Rev Dis Primers. 2020;6:90. doi: 10.1038/s41572-020-00223-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Berger M., Terrando N., Smith S.K., Browndyke J.N., Newman M.F., Mathew J.P. Neurocognitive function after cardiac surgery: from phenotypes to mechanisms. Anesthesiology. 2018;129:829–851. doi: 10.1097/aln.0000000000002194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moher D., Liberati A., Tetzlaff J., Altman D.G. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Bmj. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guyatt G.H., Oxman A.D., Vist G.E., Kunz R., Falck-Ytter Y., Alonso-Coello P., et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. Bmj. 2008;336:924–926. doi: 10.1136/bmj.39489.470347.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Luo D., Wan X., Liu J., Tong T. Optimally estimating the sample mean from the sample size, median, mid-range, and/or mid-quartile range. Stat. Methods Med. Res. 2018;27:1785–1805. doi: 10.1177/0962280216669183. [DOI] [PubMed] [Google Scholar]
- 26.Wan X., Wang W., Liu J., Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med. Res. Methodol. 2014;14:135. doi: 10.1186/1471-2288-14-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shi J., Luo D., Weng H., Zeng X.T., Lin L., Chu H., et al. Optimally estimating the sample standard deviation from the five-number summary. Res. Synth. Methods. 2020;11:641–654. doi: 10.1002/jrsm.1429. [DOI] [PubMed] [Google Scholar]
- 28.Clemmesen C.G., Lunn T.H., Kristensen M.T., Palm H., Foss N.B. Effect of a single pre-operative 125 mg dose of methylprednisolone on postoperative delirium in hip fracture patients; a randomised, double-blind, placebo-controlled trial. Anaesthesia. 2018;73:1353–1360. doi: 10.1111/anae.14406. [DOI] [PubMed] [Google Scholar]
- 29.Xiang X.B., Chen H., Wu Y.L., Wang K., Yue X., Cheng X.Q. The effect of preoperative methylprednisolone on postoperative delirium in older patients undergoing gastrointestinal surgery: a randomized, double-blind, placebo-controlled trial. J Gerontol A Biol Sci Med Sci. 2022;77:517–523. doi: 10.1093/gerona/glab248. [DOI] [PubMed] [Google Scholar]
- 30.Cho S.A., Huh I., Lee S.J., Sung T.Y., Ku G.W., Cho C.K., et al. Effects of dexamethasone on catheter-related bladder discomfort and emergence agitation: a prospective, randomized, controlled trial. Korean J Anesthesiol. 2022;75:71–78. doi: 10.4097/kja.21284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kluger M.T., Skarin M., Collier J., Rice D.A., McNair P.J., Seow M.Y., et al. Steroids to reduce the impact on delirium (STRIDE): a double-blind, randomised, placebo-controlled feasibility trial of pre-operative dexamethasone in people with hip fracture. Anaesthesia. 2021;76:1031–1041. doi: 10.1111/anae.15465. [DOI] [PubMed] [Google Scholar]
- 32.Sakic L., Tonkovic D., Godan B.J., Sakic K. The influence of dexamethasone administration in spinal anesthesia for femur fracture on postoperative cognitive dysfunction. Period. Biol. 2015;117:281–285. [Google Scholar]
- 33.Sakic L., Tonkovicc D., Hrgovic Z., Klasan A. Spinal dexamethasone effect on cognitive disorders after hip surgery. Med. Arch. 2023;77:18–23. doi: 10.5455/medarh.2023.77.18-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huang J.W., Yang Y.F., Gao X.S., Xu Z.H. A single preoperative low-dose dexamethasone may reduce the incidence and severity of postoperative delirium in the geriatric intertrochanteric fracture patients with internal fixation surgery: an exploratory analysis of a randomized, placebo-controlled trial. J. Orthop. Surg. Res. 2023;18:441. doi: 10.1186/s13018-023-03930-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moheimani H., Yaseri M. Dexamethasone reduces postoperative nausea in pediatric upper endoscopy with deep sedation: a randomized controlled trial. J. Pediatr. Gastroenterol. Nutr. 2019;69:281–286. doi: 10.1097/mpg.0000000000002398. [DOI] [PubMed] [Google Scholar]
- 36.Shama A.A.A., Elsayed A.A., Albraithen A.A., Arafa S.K. Effect of dexmedetomidine, dexamethasone, and ondansetron on postoperative nausea and vomiting in children undergoing dental rehabilitation: a randomized controlled trial. Pain Physician. 2023;26:1–11. [PubMed] [Google Scholar]
- 37.Elsonbaty M., Lsonbaty A.E. Effect of intravenous magnesium sulphate or dexamethasone as adjuvants to sevoflurane anesthesia to prevent delirium during primary cleft palate repair, controlled randomized blind study. Egypt. J. Anaesth. 2017;33:91–95. doi: 10.1016/j.egja.2016.11.003. [DOI] [Google Scholar]
- 38.Sajedi P., Baghery K., Hagibabie E., Mehr A.M. Prophylactic use of oral acetaminophen or IV dexamethasone and combination of them on prevention emergence agitation in pediatric after adenotonsillectomy. Int J Preventive Med. 2014;5:721–727. [PMC free article] [PubMed] [Google Scholar]
- 39.Khalili G., Sajedi P., Shafa A., Hosseini B., Seyyedyousefi H. A randomized evaluation of intravenous dexamethasone versus oral acetaminophen codeine in pediatric adenotonsillectomy: emergence agitation and analgesia. Middle East J Anaesthesiol. 2012;21:499–504. [PubMed] [Google Scholar]
- 40.Oh E.S., Fong T.G., Hshieh T.T., Inouye S.K. Delirium in older persons: advances in diagnosis and treatment. JAMA. 2017;318:1161–1174. doi: 10.1001/jama.2017.12067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Silva L.M., Braz L.G., Módolo N.S. Emergence agitation in pediatric anesthesia: current features. J. Pediatr. 2008;84:107–113. doi: 10.2223/jped.1763. [DOI] [PubMed] [Google Scholar]
- 42.Inouye S.K., Westendorp R.G., Saczynski J.S. Delirium in elderly people. Lancet. 2014;383:911–922. doi: 10.1016/s0140-6736(13)60688-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McDaniel M., Brudney C. Postoperative delirium: etiology and management. Curr. Opin. Crit. Care. 2012;18:372–376. doi: 10.1097/MCC.0b013e3283557211. [DOI] [PubMed] [Google Scholar]
- 44.Maldonado J.R. Pathoetiological model of delirium: a comprehensive understanding of the neurobiology of delirium and an evidence-based approach to prevention and treatment. Crit. Care Clin. 2008;24:789–856. doi: 10.1016/j.ccc.2008.06.004. ix. [DOI] [PubMed] [Google Scholar]
- 45.Awada H.N., Steinthorsdottir K.J., Schultz N.A., Hillingsø J.G., Larsen P.N., Jans Ø., et al. High-dose preoperative glucocorticoid for prevention of emergence and postoperative delirium in liver resection: a double-blinded randomized clinical trial substudy. Acta Anaesthesiol. Scand. 2022;66:696–703. doi: 10.1111/aas.14057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Orci L.A., Toso C., Mentha G., Morel P., Majno P.E. Systematic review and meta-analysis of the effect of perioperative steroids on ischaemia-reperfusion injury and surgical stress response in patients undergoing liver resection. Br. J. Surg. 2013;100:600–609. doi: 10.1002/bjs.9035. [DOI] [PubMed] [Google Scholar]
- 47.Richardson A.J., Laurence J.M., Lam V.W. Use of pre-operative steroids in liver resection: a systematic review and meta-analysis. HPB (Oxford) 2014;16:12–19. doi: 10.1111/hpb.12066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li L.Q., Wang C., Fang M.D., Xu H.Y., Lu H.L., Zhang H.Z. Effects of dexamethasone on post-operative cognitive dysfunction and delirium in adults following general anaesthesia: a meta-analysis of randomised controlled trials. BMC Anesthesiol. 2019;19:113. doi: 10.1186/s12871-019-0783-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu W., Wang Y., Wang J., Shi J., Pan J., Wang D. Effects of glucocorticoids on postoperative delirium in adult patients undergoing cardiac surgery: a systematic review and meta-analysis. Clin Ther. 2021;43:1608–1621. doi: 10.1016/j.clinthera.2021.07.021. [DOI] [PubMed] [Google Scholar]
- 50.Xie X., Gao R., Chen H., Zhang X., Cai X., Zhang C., et al. Effects of glucocorticoids on postoperative neurocognitive disorders in adult patients: a systematic review and meta-analysis. Front. Aging Neurosci. 2022;14 doi: 10.3389/fnagi.2022.939848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hovens I.B., van Leeuwen B.L., Mariani M.A., Kraneveld A.D., Schoemaker R.G. Postoperative cognitive dysfunction and neuroinflammation; Cardiac surgery and abdominal surgery are not the same. Brain Behav. Immun. 2016;54:178–193. doi: 10.1016/j.bbi.2016.02.003. [DOI] [PubMed] [Google Scholar]
- 52.Aldecoa C., Bettelli G., Bilotta F., Sanders R.D., Audisio R., Borozdina A., et al. European Society of Anaesthesiology evidence-based and consensus-based guideline on postoperative delirium. Eur. J. Anaesthesiol. 2017;34:192–214. doi: 10.1097/eja.0000000000000594. [DOI] [PubMed] [Google Scholar]
- 53.Reiss R., Deutsch A., Nudelman I. Surgical problems in octogenarians: epidemiological analysis of 1,083 consecutive admissions. World J. Surg. 1992;16:1017–1020. doi: 10.1007/bf02067023. discussion 20-1. [DOI] [PubMed] [Google Scholar]
- 54.The DSM-5 criteria, level of arousal and delirium diagnosis: inclusiveness is safer. BMC Med. 2014;12:141. doi: 10.1186/s12916-014-0141-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bajwa S.A., Costi D., Cyna A.M. A comparison of emergence delirium scales following general anesthesia in children. Paediatr. Anaesth. 2010;20:704–711. doi: 10.1111/j.1460-9592.2010.03328.x. [DOI] [PubMed] [Google Scholar]
- 56.Seguro L.P., Rosario C., Shoenfeld Y. Long-term complications of past glucocorticoid use. Autoimmun. Rev. 2013;12:629–632. doi: 10.1016/j.autrev.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 57.Polderman J.A., Farhang-Razi V., Van Dieren S., Kranke P., DeVries J.H., Hollmann M.W., et al. Adverse side effects of dexamethasone in surgical patients. Cochrane Database Syst. Rev. 2018;11:Cd011940. doi: 10.1002/14651858.CD011940.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vlajkovic G.P., Sindjelic R.P. Emergence delirium in children: many questions, few answers. Anesth. Analg. 2007;104:84–91. doi: 10.1213/01.ane.0000250914.91881.a8. [DOI] [PubMed] [Google Scholar]
- 59.Mason K.P. Paediatric emergence delirium: a comprehensive review and interpretation of the literature. Br. J. Anaesth. 2017;118:335–343. doi: 10.1093/bja/aew477. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
As this research is a meta-analysis of previous data, no new data were generated.