Abstract
Objective
To investigate the effectiveness of 12-weeks hybrid virtual coaching on health-related quality-of-life (HrQoL) in patients with stable COPD.
Methods
We equipped all patients with a CAir Desk for telemonitoring, the intervention group additionally received hybrid virtual coaching through the built-in smartphone. The multimodal intervention based on the Living well with COPD programme, containing educational content, physical activity coaching, and home-based exercises. Primary outcome was HrQoL as measured by the SGRQ. Secondary outcomes were symptom burden, physical activity, functional exercise capacity, and lung function. Between-group differences were calculated using linear regression models, controlling for baseline differences.
Results
We included 30 participants with COPD (13/17 women/men; 63 [9] years; FEV1 54 [22] % predicted), 24 (80%) completed the study. SGRQ improved in both groups (intervention: −4.5 [20.1]; control: −2.7 [7.4] points) without statistically significant or clinically relevant between-group differences (B = −2.5 points, 95% CI = −24.3, 19.3, p = 0.81). Physical activity increased only in the intervention group (313 [1561] vs −364 [2399] steps) without statistically significant but clinically relevant between-group difference (B = 2147 steps, 95% CI = −86, 4395, p = 0.06). Symptom burden decreased in both groups (−4.2 [6.7] vs −1.0 [2.8] points) without statistically significant but clinically relevant between-group difference (B = −3.0 points, 95% CI = −10.8, 5.0, p = 0.43).
Conclusion
Twelve-weeks hybrid virtual coaching did not improve HrQoL more than telemonitoring only in patients with stable COPD. The intervention group improved their physical activity and symptom burden clinically relevant more than the control group.
Keywords: COPD, conversational agent, telemedicine, physical activity
Introduction
Chronic Obstructive Pulmonary Disease (COPD) poses a significant healthcare burden which is projected to increase further in the coming decade.1 Far more patients need multimodal treatment alleviating their symptoms than the current healthcare systems can manage. Thus, novel technologies may be a potent option to extend accessibility to care for patients with COPD.
After initial diagnosis and establishment of pharmacological treatment for COPD, the focus of care is on improving or maintaining health-related quality-of-life (HrQoL) and physical functioning.2,3 It is well established that multimodal interventions are effective in achieving that goal.2 However, multimodal treatment is resource intensive, requiring a broad range of specialised healthcare practitioners (ie, physicians, nurses, physiotherapists, nutritionists) and considerable time commitment from patients. Furthermore, access to multimodal interventions, typically available only at large centralized units, is often limited by logistical challenges such as travel, geographical distance, and programme scheduling, as well as patient symptoms like dyspnoea and anxiety. These barriers result in participation rates as low as 10% of eligible individuals.4 Recent advances in healthcare research suggest sophisticated technical solutions such as remote monitoring and sensing, virtual and hybrid communication, and tele-rehabilitation as promising.5,6 These approaches have the potential of reaching a broad range of patients in a time-asynchronous manner and at scalable cost.
Promising data on the effectiveness of tele-rehabilitation studies and evidence that patients with COPD are interested in digital solutions and adhere highly are encouraging to move this line of research forward.7 Digital solutions that include interactive elements such as virtual coaching solutions in the form of conversational agents (CA) are a particularly patient-engaging way to implement tele-rehabilitation and are increasingly investigated in clinical populations.8–10
Nevertheless, comprehensive digital multimodal interventions remain scarcely applied and investigated in chronic respiratory disease.11 Reasons for this may be the relatively low number of validated remote sensors and the requirement of a multidisciplinary team to develop and establish the intervention.5 To the best of our knowledge, no studies are available implementing CAs in multimodal interventions for COPD.
We aimed to investigate the effectiveness of a 12-week hybrid virtual coaching solution on HrQoL with telemonitoring of clinical parameters, delivered through the CAir Desk,7,12 in patients with stable COPD.
Methods
Study Design
We conducted a single-centre, two-arm randomized, single (assessor)-blind study at the University Hospital Zurich, Switzerland. The study ran from February 2021 until completion in May 2022.
We conducted this study in accordance with the Declaration of Helsinki,13 the principles of Good Clinical Practice, the Human Research Act (HRA),14 and the Ordinance on Human Research with the Exception of Clinical trials (HRO).15 All subjects provided written informed consent. The Ethics Committee of the Canton of Zurich approved the study (EK-ZH-NR: 2020–00707), and the study is registered on www.ClinicalTrials.gov (NCT04373070). This manuscript is in accordance with the Consolidated Standards of Reporting Trials (CONSORT).16
Study Participants
Patients with a COPD diagnosis according to the Global Initiative for Chronic Obstructive Lung Disease (GOLD)2 and ≥40 years were deemed eligible for this study. We excluded participants in case of: recent acute COPD exacerbation (ie, within the last 6 weeks), pulmonary rehabilitation within the last 3 months, and pregnancy. In addition, patients needed to be fluent in German, since this was the language of communication with the CA.
Experimental Procedures
All participants received a CAir Desk for home-monitoring for 12 weeks, their usual care (ie, physician visits, prescribed medication) was not altered. A researcher not involved in the study procedures randomised participants on a 1:2 (control: intervention) ratio using computerised randomisation with permuted random block sizes of 2 or 4. The assessor collecting baseline and follow-up data was blinded to group allocation.
The intervention consisted of a hybrid virtual coaching solution which was based on the Living well with COPD programme (http://www.livingwellwithcopd.com). Home-monitoring was done independent of group allocation. Thus, the groups differed solely in terms of the hybrid virtual coaching solution. The published study protocol provides a study overview including details on the baseline and follow-up testing days.12
The CAir Desk
The CAir Desk is a multisensory home-monitoring system and was developed and tested for chronic respiratory diseases.7,12 For the present study, the CAir Desks consisted of a smartphone (Galaxy A320, Samsung Electronics, South Korea), a wrist-worn physical activity (PA) monitor (Charge 3, Fitbit, USA), a cordless hand-spirometry device (Air Next, NuvoAir, Sweden), a sputum tray (custom-made), and an environmental air quality monitor (Foobot, Airboxlab, Luxembourg). Details on the devices used, including the CAir mobile phone app, and the cloud backend are outlined in the published study protocol.12
Programme Components
Programme details are given in the published study protocol.12 Beneath, the intervention is summarised in brief with relevant additional specifications.
Physical Activity Coaching
Patients randomised to the intervention group received feedback on their daily step count through the CA. The CA coached the patients towards increasing their daily step count by 15%, a commonly used threshold with proven benefits in patients with COPD.17–19 The PA coaching started with the second week, using the first week as baseline measurements.
Educational Programme
The Living well with COPD educational programme was delivered virtually via the CA and only accessible for the intervention group. The educational content addressed comprehensive topics tailored for patients with COPD, ranging from coping during activities of daily living to travel advice.
The interactions were self-paced with predefined answer options. Its conversation style was based on previous studies on the preferences of patients with COPD.20 There were regular sessions during which the participants could engage in a chat with a study physician, making the conversation hybrid. Finally, study staff called all patients in study weeks 1, 4, and 6 to guarantee technical functionality.
Home-Based Exercise
The CA provided the participants in the intervention group with a structured home-based exercise training programme. The programme incorporated strengthening exercises for the whole body, stretching exercises, and breathing exercises. For some of the strengthening exercises, an elastic training band was required, which we provided with the CAir Desk. The CA offered the patients to exercise 6 days per week with one rest day. The patients could start training sessions immediately or delay them to a later time during the day. The CA guided the exercises with concise descriptions and videos. After completing an exercise, the patients provided the CA with the number of repetitions achieved and a rating of perceived exertion.
For each session, the exercises were combined randomly from a pool of exercises. Each session consisted of two strengthening exercises and either one stretching or one breathing exercise. Adhering to the exercise principle of progressive overload, the CA instructed the patients to perform as many repetitions as possible (for the strengthening exercises) and proposed progressions.21
Study Outcomes
Participants attended one baseline and one follow-up visit. The baseline visit incorporated testing of lung function, functional exercise capacity, and completion of questionnaires. In addition, the patients received their CAir Desk along with an introduction to its functionalities. The tests with the follow-up visit were identical, and patients were asked to complete a questionnaire on the usability of the CAir Desk. Vast details on the study outcomes are given in the published study protocol.12
Primary Outcome
The primary outcome was the St. George Respiratory Questionnaire (SGRQ).22 The minimal clinical important difference (MCID) for the SGRQ is considered 4 points.23
Secondary Outcomes
Physical Activity
We used daily step count as a marker for PA. The reportings on MCID in daily step count in patients with COPD are rather heterogeneous.3 Thus, we applied a conservative MCID of 1000 steps/day to our analysis.
Functional Exercise Capacity
At the baseline and the follow-up visit, we assessed functional exercise capacity with the 6-minute walk test (6MWT) and the 1-minute sit-to-stand test (1MSTST).24,25 The MCID for the 6MWT is considered 30m,24 and for the 1MSTST 3 repetitions.26
Spirometry
At baseline and follow-up visits, patients underwent a spirometry test with diffusion capacity measurements.27,28 With home-monitoring, patients recorded daily spirometries on the CAir Desk. They were trained in these measurements at the baseline visit and asked to record 3 reproducible measurements every day around the same time.
COPD Related Symptom Burden
We assessed symptom burden with the COPD Assessment Test (CAT).29 Patients were asked to complete the questionnaire at baseline and follow-up visits. In addition, the CA asked the patients to fill the CAT every evening at bedtime, rating the symptoms of the current day.30 The MCID for the CAT is considered 2 points.31
Perceptions on the Programme
Patients filled a purpose-designed questionnaire at the end of their study participation concerning perceptions on the programme.
Statistical Analysis
Sample size estimation suggested to include 42 participants, including a generous account of dropouts.12 Data was analysed on an intention-to-treat basis. Distribution of variables was determined visually using quantile–quantile plots and showed normality. Group characteristics and baseline measurements are presented using descriptive statistics. Differences between groups at the primary and secondary endpoints were calculated using linear regression models, controlling for baseline differences in PA and 1MSTST. No corrections for multiple testing were applied since no statistically significant results were found.
Data extraction and preprocessing was done with Python version 3.12.3 for Windows (Python Software Foundation, USA). Statistical analyses were done with R version 4.2.3 for Windows (R Core Team 2023, R Foundation for Statistical Computing, Austria).
Results
Participant Characteristics
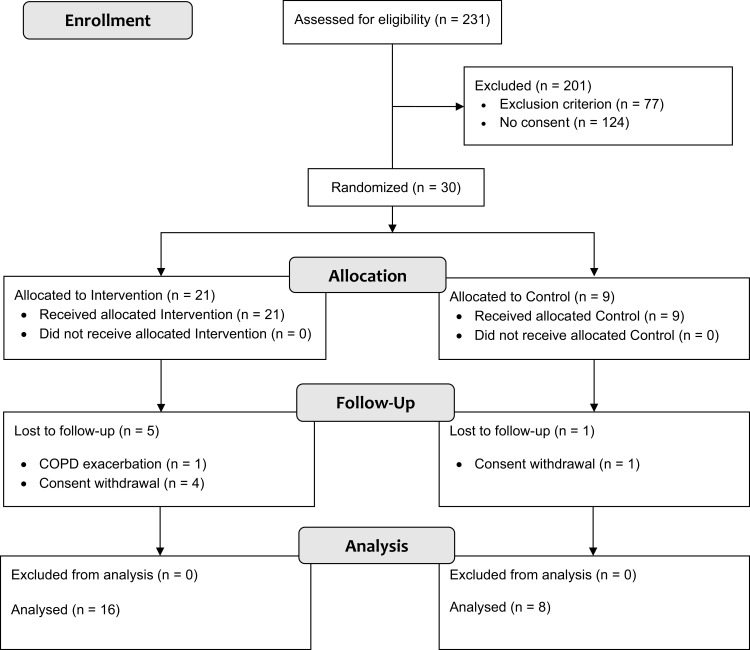
We included 30 participants, of whom 24 (80%) completed the study, see Figure 1. The sample had a mean (standard deviation, SD) age of 63 (9) years, and a forced expiratory volume in 1 second (FEV1) of 54 (22) % predicted. See Table 1 for detailed baseline characteristics stratified by group allocation. One participant in the intervention group experienced an acute exacerbation of their COPD, no adverse and serious adverse events related to the intervention occurred.
Figure 1.
Study participant flow chart.
Table 1.
Participant Characteristics at Baseline
| Overall | Intervention | Control | |
|---|---|---|---|
| N | 30 | 21 | 9 |
| Age, years | 63 (9) | 64 (9) | 61 (9) |
| Sex, female/male (%) | 13/17 (43/57) | 9/12 (43/57) | 4/5 (44/56) |
| GOLD stage, n (%) | |||
| 1 | 3 (10) | 1 (5) | 2 (22) |
| 2 | 14 (47) | 13 (62) | 1 (11) |
| 3 | 9 (30) | 4 (19) | 5 (56) |
| 4 | 4 (13) | 3 (14) | 1 (11) |
| COPD Risk Group, n (%) | |||
| A | 8 (27) | 6 (29) | 2 (22) |
| B | 13 (43) | 10 (48) | 3 (33) |
| E | 9 (30) | 5 (24) | 4 (44) |
| SGRQ, points | 42.1 (15.6) | 43.3 (15.5) | 39.5 (16.2) |
| CAT, points | 14.7 (6.1) | 15.5 (6.3) | 12.9 (5.3) |
| FEV1, l | 1.5 (0.6) | 1.6 (0.6) | 1.4 (0.6) |
| FEV1, % predicted | 54 (22) | 56 (22) | 48 (23) |
| FVC, l | 2.9 (0.8) | 3.0 (0.9) | 2.9 (0.7) |
| FVC, % predicted | 78 (20) | 79 (20) | 76 (20) |
| TLco, mL/mmHg/min | 4.5 (1.6) | 4.6 (1.7) | 4.4 (1.5) |
| TLco, % predicted | 54 (20) | 55 (21) | 51 (19) |
| 1MSTST, repetitions | 27 (9) | 25 (9) | 30 (8) |
| 6MWT, m | 465 (81) | 471 (92) | 450 (51) |
| Steps per day, n | 8140 (4089) | 8782 (3027) | 7016 (5558) |
Note: Data are mean (SD) or n (%).
Abbreviations: SGRQ, St. George Respiratory Questionnaire; CAT, COPD Assessment Test; FEV1, forced expiratory volume in one second; FVC, forced vital capacity; TLco, transfer factor of the lung for carbon monoxide; 1MSTST, 1-minute sit-to-stand test; 6MWT, 6-minute walk test.
St. George Respiratory Questionnaire
The SGRQ total score decreased by mean (SD) −4.5 (20.1) points, from 42.3 (14.1) to 37.0 (13.4) points in the intervention group and by −2.7 (7.4) points, from 42.0 (15.3) to 39.9 (15.5) points in the control group. The between-group difference of the change corrected for baseline differences was not statistically significant (B = −2.5 points, 95% CI = −24.3, 19.3, p = 0.81). Three (14%) participants improved beyond the MCID in the intervention group, while one (11%) did in the control group.
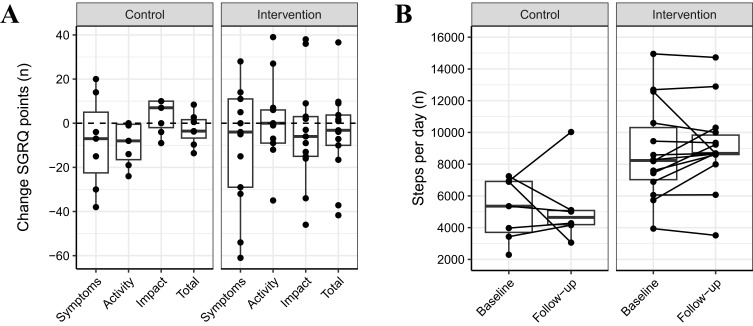
Data on the SGRQ subcategories is shown in Table 2 and Figure 2A.
Table 2.
Changes in Questionnaire Scores Across Study Groups
| Intervention | Control | Between Groups | |||||
|---|---|---|---|---|---|---|---|
| Pre | Post | Delta | Pre | Post | Delta | p-value | |
| St. George Respiratory Questionnaire | |||||||
| Symptoms | 47.0 (25.8) | 38.9 (19.1) | −9.9 (27.1) | 49.5 (25.4) | 43.9 (14.8) | −8.6 (21.3) | 0.81 |
| Activity | 57.4 (9.5) | 58.1 (13.6) | 0.7 (18.3) | 66.0 (17.4) | 57.4 (20.7) | −9.4 (9.8) | 0.38 |
| Impact | 32.2 (17.9) | 26.4 (19.3) | −4.2 (23.6) | 26.6 (12.8) | 29.9 (14.8) | 3.4 (7.8) | 0.49 |
| Total | 42.3 (14.1) | 37.0 (13.4) | −4.5 (20.1) | 42.0 (15.3) | 39.9 (15.5) | −2.7 (7.4) | 0.81 |
| COPD Assessment Test | |||||||
| Score | 15.3 (6.1) | 11.3 (6.6) | −4.2 (6.7) | 14.0 (4.4) | 11.6 (2.0) | −1.0 (2.8) | 0.43 |
Notes: Data are mean (SD). The p-values are calculated with linear regression modelling adjusted for baseline differences in daily step count and 1-minute sit-to-stand test repetitions.
Figure 2.
Effects on SGRQ and physical activity stratified by group. (A) shows the changes in total SGRQ score and all subdomains, (B) shows the individual courses from baseline to follow-up in daily step count.
Abbreviation: SGRQ, St. George Respiratory Questionnaire.
Physical Activity
Daily step count increased by mean (SD) 313 (1561) steps, from 8782 (3027) to 9095 (2645) steps in the intervention group and decreased by −364 (2399) steps, from 7016 (5558) to 5274 (2442) steps in the control group, see Table 3 and Figure 2B. The between-group difference of the change corrected for baseline differences was not statistically significant (B = 2147 steps, 95% CI = −86, 4395, p = 0.06). Five (24%) participants improved beyond the MCID in the intervention group, while one (11%) did in the control group.
Table 3.
Changes in Functional Exercise Capacity and Physical Activity Across Study Groups
| Intervention | Control | Between Groups | |||||
|---|---|---|---|---|---|---|---|
| Pre | Post | Delta | Pre | Post | Delta | p-value | |
| Functional exercise capacity | |||||||
| 6MWT, m | 481 (82) | 511 (48) | 23 (39) | 458 (48) | 476 (54) | 17 (57) | 0.61 |
| 1MSTST, repetitions | 27 (8) | 30 (9) | 3 (6) | 31 (8) | 31 (8) | 2 (9) | 0.80 |
| Physical activity | |||||||
| Daily step count, n | 8782 (3027) | 9095 (2645) | 313 (1561) | 7016 (5558) | 5274 (2442) | −364 (2399) | 0.06 |
Notes: Data are mean (SD). The p-values are calculated with linear regression modelling adjusted for baseline differences in daily step count and 1MSTST repetitions.
Abbreviations: 6MWT, 6-minute walk test; 1MSTST, 1-minute sit-to-stand test.
Functional Exercise Capacity
6MWT distance increased by mean (SD) 23 (39) m, from 481 (82) to 511 (48) m in the intervention group and by 17 (57) m, from 458 (48) to 476 (54) m in the control group, see Table 3. The between-group difference of the change corrected for baseline differences was not statistically significant (B = 11.6 m, 95% CI = −35.8, 58.9, p = 0.61). Six (29%) participants improved beyond the MCID in the intervention group, while two (22%) did in the control group.
1MSTST repetitions increased by 3 (6), from 27 (8) to 30 (9) in the intervention group and by 2 (9), from 31 (8) to 31 (8) in the control group, see Table 3. The between-group difference of the change corrected for baseline differences was not statistically significant (B = −0.8 repetitions, 95% CI = −7.4, 5.8, p = 0.80). Eight (38%) participants improved beyond the MCID in the intervention group, while two (22%) did in the control group.
COPD Related Symptom Burden
CAT scores decreased by mean (SD) −4.2 (6.7) points, from 15.3 (6.1) to 11.3 (6.6) points in the intervention group and by −1.0 (2.8) points, from 14.0 (4.4) to 11.6 (2.0) points in the control group, see Table 2. The between-group difference of the change corrected for baseline differences was not statistically significant (B = −3.0 points, 95% CI = −10.8, 5.0, p = 0.43). Three (14%) participants improved beyond the MCID in the intervention group, while one (11%) did in the control group.
Spirometry
Lung function remained stable in both groups over the course of the study. At the follow-up, the intervention group had an FEV1 of mean (SD) 55 (23), forced vital capacity (FVC) of 76 (21), and a transfer factor of the lung for carbon monoxide (TLco) of 56 (23)% predicted. The control group had an FEV1 of 44 (24), FVC of 73 (22), and TLco of 49 (19)% predicted.
Participant Adherence and Experiences
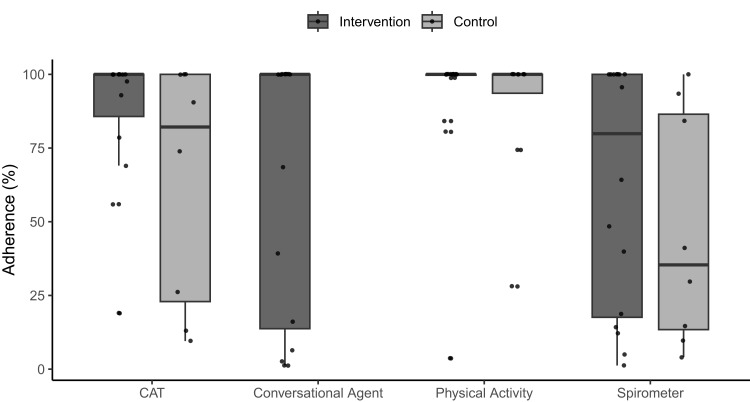
The patients in the intervention group completed 65 (44) % of the CA interactions. Regarding PA measurements, the intervention group collected valid data on mean (SD) 92 (24) % of study days, while the control group did on 89 (26) %. The intervention group completed the CAT questionnaire on 88 (23) % of study days, while the control group did on 64 (41) %. The intervention group collected complete spirometries (ie, 3 reproducible manoeuvres) on 62 (41) % of study days, while the control group did on 47 (40) %. The adherence data is displayed in Figure 3.
Figure 3.
Adherence per sensor, stratified by group and as individual data. The adherence is quantified as the percentage of all interactions completed (conversational agent) and the percentage of valid measurement days (physical activity monitor, CAT, and spirometer).
Abbreviation: CAT, COPD Assessment Test.
Patients in the intervention group estimated to have spent 15 (13) min per day interacting with the CAir Desk. In the control group, patients estimated to have spent 13 (5) min per day conducting the home-monitoring.
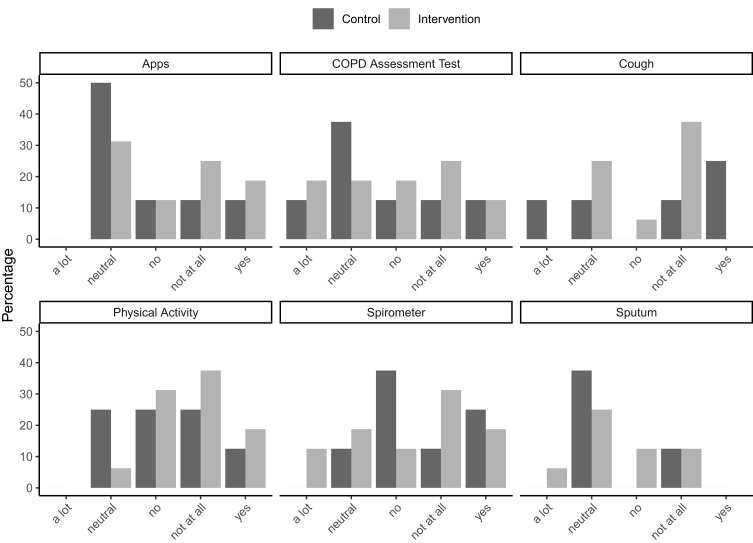
Most patients in both groups did not indicate that they were bothered by the daily sensor interactions, see Figure 4. In both groups, the sensors that required minimal or no interaction (ie, cough recording and PA monitor) scored best.
Figure 4.
Patient satisfaction with all components of the CAir Desk in percentages. The participants were asked: “How bothering was it for you to conduct the measurements with [sensor] in your everyday life?”. They were asked to leave the question blank (ie, NA) if they did not use a specific sensor.
Discussion
We report on the effectiveness of a multimodal intervention delivered in a hybrid virtual manner including home-monitoring for patients with stable COPD. Our study showed that patients with COPD receiving the intervention did not increase their HrQoL significantly more than patients participating in home-monitoring only. However, only patients receiving the hybrid virtual intervention increased their PA to a clinically relevant degree, while patients in the control group showed a decrease.
Although both groups increased their HrQoL, as quantified by the SGRQ, the intervention group did not show superior increases compared to the control group. In addition, the improvements in both groups may not be considered clinically relevant. Earlier studies using digital coaching approaches showed conflicting results regarding HrQoL. While one study achieved substantial and relevant increases,32 another one did not show between-group differences.33 Nevertheless, delivering the Living well with COPD programme in face-to-face methodology has shown superior improvements in HrQoL to usual care only.34 Thus, it seems reasonable that face-to-face delivery of health information proves more impactful than through a CA. Possibly because a face-to-face interaction with a healthcare practitioner allows patients to have their individual questions answered immediately. We acknowledged this discrepancy between the modalities in the planning stage of this RCT and implemented the patient-physician chat sessions. However, chat sessions seem not to compensate for the lack of interpersonal communication.
In line with the 4-month results of Moy et al,33 who used a web-based interface for PA coaching and pedometer-based feedback without CA implementation, we found clinically relevant increases in daily step count in the intervention group only. Interestingly, the mentioned study showed that PA, valid pedometer days, and number of web-interface logins decreased when the programme did not provide new educational content. We believe that CAs may provide an appealing solution here with the possibility of reminders and tailored interaction facilitating adherence, as shown in various populations.35 However, this remains to be investigated in patients with COPD.
The intervention reduced COPD-related symptom burden clinically relevant more than home-monitoring only. This was a surprising finding given the findings in the SGRQ. However, reductions in COPD-related symptom burden can be achieved by exercise and PA interventions which our multimodal programme also included.36,37
We did not find any between-group differences in functional exercise capacity. However, more patients improved their 1MSTST to a clinically relevant degree when receiving the intervention (38% vs 22%). While delivering an exercise programme with a CA is powerful and convenient in terms of instructional methods (we used video and text-based instructions), exercises must be delivered in a “one-size-fits-all” approach. Individual tailoring to every patient’s needs and goals is not possible, and we think that the results reflect this. Our exercise programme may not fulfil the exercise criteria for specificity and progressive overload.38 However, we still advocate for general exercise in digital multimodal interventions, emphasising on the importance of movement and PA in the treatment of COPD and familiarising patients with a regular exercise routine. Nevertheless, when the primary goal is an improvement of endurance or strength, a personalised approach should be chosen, which may still be delivered in a tele-setting.11
The patients in the intervention group showed higher adherence to the daily measurements than the control group and the adherence to the CA interactions was high with some exceptions. Interestingly, adherence to the chatbot was either close to maximal or the minimum. Only two patients showed medium adherence with between 30 and 70% completed interactions. Patient experiences with the CAir Desk and the CA were positive and comparable to our preliminary study.7 Interestingly, patients in the intervention group indicated a similar amount of time dedicated to interacting with the CAir Desk as in the feasibility study, despite the additional CA component. In line with our findings from the feasibility study and other research, patients with COPD show high interest and have advanced skills in handling digital solutions.5,7,39
This study has some limitations; first, our randomisation showed baseline differences between groups, for which we applied statistical adjustment. Second, we concluded participant recruitment before reaching the pre-determined 42 participants. This was because the large dropout rate we assumed during study planning turned out to be inaccurate. Thus, our study has the expected and pre-calculated power.
In conclusion, a 12-week hybrid virtual coaching intervention with telemonitoring did not show improvements in HrQoL superior to telemonitoring only in patients with stable COPD. Patients in the intervention group improved their PA and their symptom burden to a clinically relevant degree, while the control group did not show relevant changes. Accordingly, hybrid virtual coaching seems to be effective in improving PA and symptom burden but not HrQoL in patients with stable COPD.
Acknowledgments
We thank our study participants for dedicating their time to research. We thank Alexandra Arvaji with the Department of Pulmonology, University Hospital Zurich for assistance with baseline data acquisition and storage. We thank Diego M. Baur, PhD with the Department of Pulmonology, University Hospital Zurich for his assistance with the visualisations for this work.
Funding Statement
This study was funded by Innosuisse, The Swiss Innovation Agency (grant number: 29844.1).
Abbreviations
COPD, Chronic Obstructive Pulmonary Disease; HrQoL, Health-related quality-of-life; CA, Conversational agent; HRA, Human Research Act; HRO, Ordinance on Human Research with the Exception of Clinical Trials; CONSORT, Consolidated Standards of Reporting Trials; GOLD, Global Initiative for Chronic Obstructive Lung Disease; SGRQ, St. George Respiratory Questionnaire; MCID, Minimal clinical important difference; PA, Physical activity; 6MWT, 6-minute walk test; 1MSTST, 1-minute sit-to-stand test; CAT, COPD Assessment Test; FEV1, Forced expiratory volume in 1 second; FVC, Forced vital capacity; TLco, Transfer factor of the lung for carbon monoxide.
Data Sharing Statement
Data generated in this study is available upon reasonable request directed to the corresponding author.
Ethics Statement
The Ethics Committee of the Canton of Zurich approved the study (EK-ZH-NR: 2020-00707).
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
D. Kohlbrenner, M. Kuhn, A. Kläy, N.A. Sievi, M. Muszynski, A. Ivankay, C.S. Gross, A. Asisof, T. Brunschwiler have nothing to disclose. C.F. Clarenbach reports advisory fees from AstraZeneca, Boehringer Ingelheim, CSL Behring, Daiichi Synkyo, GlaxoSmithKline, Novartis, Sanofi, OM Pharma, MSD, Grifols, Vifor, all outside the submitted work.
References
- 1.Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006;3(11):e442. doi: 10.1371/journal.pmed.0030442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease (2024 Report). 2024.
- 3.Watz H, Pitta F, Rochester CL, et al. An official European Respiratory Society statement on physical activity in COPD. Eur Respir J. 2014;44(6):1521–1537. doi: 10.1183/09031936.00046814 [DOI] [PubMed] [Google Scholar]
- 4.Cox NS, Oliveira CC, Lahham A, Holland AE. Pulmonary rehabilitation referral and participation are commonly influenced by environment, knowledge, and beliefs about consequences: a systematic review using the theoretical domains framework. J Physiother. 2017;63(2):84–93. doi: 10.1016/j.jphys.2017.02.002 [DOI] [PubMed] [Google Scholar]
- 5.Pinnock H, Poberezhets V, Drummond D, editors. Digital Respiratory Healthcare. European Respiratory Society; 2023. [Google Scholar]
- 6.Cox NS, Dal Corso S, Hansen H, et al. Telerehabilitation for chronic respiratory disease. Cochrane Database Syst Rev. 2021;1(1):CD013040. doi: 10.1002/14651858.CD013040.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kohlbrenner D, Clarenbach CF, Ivankay A, et al. Multisensory home-monitoring in individuals with stable chronic obstructive pulmonary disease and asthma: usability study of the CAir-desk. JMIR Hum Factors. 2022;9(1):e31448. doi: 10.2196/31448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ulrich S, Gantenbein AR, Zuber V, Von Wyl A, Kowatsch T, Kunzli H. Development and evaluation of a smartphone-based chatbot coach to facilitate a balanced lifestyle in individuals with headaches (BalanceUP App): randomized controlled trial. J Med Internet Res. 2024;26:e50132. doi: 10.2196/50132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Livermore P, Kupiec K, Wedderburn LR, et al. Designing, developing, and testing a chatbot for parents and caregivers of children and young people with rheumatological conditions (the IMPACT study): protocol for a co-designed proof-of-concept study. JMIR Res Protoc. 2024;13:e57238. doi: 10.2196/57238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Collins L, Nicholson N, Lidbetter N, Smithson D, Baker P. Implementation of anxiety UK’s ask anxia chatbot service: lessons learned. JMIR Hum Factors. 2024;11:e53897. doi: 10.2196/53897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Janjua S, Banchoff E, Threapleton CJ, Prigmore S, Fletcher J, Disler RT. Digital interventions for the management of chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2021;4(4):CD013246. doi: 10.1002/14651858.CD013246.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gross C, Kohlbrenner D, Clarenbach CF, et al. A telemonitoring and hybrid virtual coaching solution “CAir” for patients with chronic obstructive pulmonary disease: protocol for a randomized controlled trial. JMIR Res Protoc. 2020;9(10):e20412. doi: 10.2196/20412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Declaration of Helsinki. Available from: https://www.wma.net/policies-post/wma-declaration-of-helsinki-ethical-principles-for-medical-research-involving-human-subjects/. Accessed December 18, 2024.
- 14.Human Research Act (HRA). Available from: https://www.admin.ch/opc/de/classified-compilation/20061313/index.html. Accessed December 18, 2024.
- 15.Ordinance on human research with the exception of clinical trials (HRO). Available from: https://www.admin.ch/opc/en/classified-compilation/20121177/index.html. Accessed December 18, 2024.
- 16.Schulz KF, Altman DG, Moher D; The CONSORT Group. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMJ. 2010;340:c332. doi: 10.1136/bmj.c332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kohlbrenner D, Sievi NA, Senn O, Kohler M, Clarenbach CF. Long-term effects of pedometer-based physical activity coaching in severe COPD: a randomized controlled trial. Int J Chron Obstruct Pulmon Dis. 2020;15:2837–2846. doi: 10.2147/COPD.S279293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kohlbrenner D, Clarenbach CF, Thiel S, Roeder M, Kohler M, Sievi NA. A few more steps lead to improvements in endothelial function in severe and very severe COPD. Respir Med. 2021;176:106246. doi: 10.1016/j.rmed.2020.106246 [DOI] [PubMed] [Google Scholar]
- 19.Kuhn M, Kohlbrenner D, Sievi NA, Clarenbach CF. Increasing daily physical activity and its effects on QTc time in severe to very severe COPD: a secondary analysis of a randomised controlled trial. COPD. 2022;19(1):339–344. doi: 10.1080/15412555.2022.2101992 [DOI] [PubMed] [Google Scholar]
- 20.Gross C, Schachner T, Hasl A, et al. Personalization of conversational agent-patient interaction styles for chronic disease management: two consecutive cross-sectional questionnaire studies. J Med Internet Res. 2021;23(5):e26643. doi: 10.2196/26643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.American College of Sports Medicine. American College of Sports Medicine position stand. Progression models in resistance training for healthy adults. Med Sci Sports Exerc. 2009;41(3):687–708. doi: 10.1249/MSS.0b013e3181915670 [DOI] [PubMed] [Google Scholar]
- 22.Jones PW, Quirk FH, Baveystock CM. The St. George’s Respiratory Questionnaire. Respir Med. 1991;85(Suppl B):25–31. discussion 27–33. doi: 10.1016/S0954-6111(06)80166-6 [DOI] [PubMed] [Google Scholar]
- 23.Jones PW. St. George’s Respiratory Questionnaire: MCID. COPD. 2005;2(1):75–79. doi: 10.1081/COPD-200050513 [DOI] [PubMed] [Google Scholar]
- 24.Holland AE, Spruit MA, Troosters T, et al. An official European Respiratory Society/American Thoracic Society technical standard: field walking tests in chronic respiratory disease. Eur Respir J. 2014;44(6):1428–1446. doi: 10.1183/09031936.00150314 [DOI] [PubMed] [Google Scholar]
- 25.Crook S, Busching G, Schultz K, et al. A multicentre validation of the 1-min sit-to-stand test in patients with COPD. Eur Respir J. 2017;49(3):1601871. doi: 10.1183/13993003.01871-2016 [DOI] [PubMed] [Google Scholar]
- 26.Vaidya T, de Bisschop C, Beaumont M, et al. Is the 1-minute sit-to-stand test a good tool for the evaluation of the impact of pulmonary rehabilitation? Determination of the minimal important difference in COPD. Int J Chron Obstruct Pulmon Dis. 2016;11:2609–2616. doi: 10.2147/COPD.S115439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Graham BL, Steenbruggen I, Miller MR, et al. Standardization of spirometry 2019 update. An official American Thoracic Society and European Respiratory Society technical statement. Am J Respir Crit Care Med. 2019;200(8):e70–e88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Graham BL, Brusasco V, Burgos F, et al. 2017 ERS/ATS standards for single-breath carbon monoxide uptake in the lung. Eur Respir J. 2017;49(1):1600016. doi: 10.1183/13993003.00016-2016 [DOI] [PubMed] [Google Scholar]
- 29.Jones PW, Harding G, Berry P, Wiklund I, Chen WH, Kline Leidy N. Development and first validation of the COPD assessment test. Eur Respir J. 2009;34(3):648–654. doi: 10.1183/09031936.00102509 [DOI] [PubMed] [Google Scholar]
- 30.Xiao Z, Muszynski M, Marcinkevičs R, et al. Breathing new life into COPD assessment: multisensory home-monitoring for predicting severity. In: International Conference on Multimodal Interaction. 2023. [Google Scholar]
- 31.Kon SS, Canavan JL, Jones SE, et al. Minimum clinically important difference for the COPD assessment test: a prospective analysis. Lancet Respir Med. 2014;2(3):195–203. doi: 10.1016/S2213-2600(14)70001-3 [DOI] [PubMed] [Google Scholar]
- 32.Wang L, He L, Tao Y, et al. Evaluating a web-based coaching program using electronic health records for patients with chronic obstructive pulmonary disease in china: randomized controlled trial. J Med Internet Res. 2017;19(7):e264. doi: 10.2196/jmir.6743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moy ML, Martinez CH, Kadri R, et al. Long-term effects of an internet-mediated pedometer-based walking program for chronic obstructive pulmonary disease: randomized controlled trial. J Med Internet Res. 2016;18(8):e215. doi: 10.2196/jmir.5622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bourbeau J, Julien M, Maltais F, et al. Reduction of hospital utilization in patients with chronic obstructive pulmonary disease: a disease-specific self-management intervention. Arch Intern Med. 2003;163(5):585–591. doi: 10.1001/archinte.163.5.585 [DOI] [PubMed] [Google Scholar]
- 35.Luo TC, Aguilera A, Lyles CR, Figueroa CA. Promoting physical activity through conversational agents: mixed methods systematic review. J Med Internet Res. 2021;23(9):e25486. doi: 10.2196/25486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McCarthy B, Casey D, Devane D, Murphy K, Murphy E, Lacasse Y. Pulmonary rehabilitation for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2015;2015(2):CD003793. doi: 10.1002/14651858.CD003793.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maltais F, Decramer M, Casaburi R, et al. An official American Thoracic Society/European Respiratory Society statement: update on limb muscle dysfunction in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2014;189(9):e15–62. doi: 10.1164/rccm.201402-0373ST [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liguori G, Feito Y, Fountaine C, Roy B. ACSM’s Guidelines for Exercise Testing and Prescription. 11th ed. Philadelphia: Wolters Kluwer; 2021. [Google Scholar]
- 39.Sonnerfors P, Skavberg Roaldsen K, Stahle A, Wadell K, Halvarsson A. Access to, use, knowledge, and preferences for information technology and technical equipment among people with chronic obstructive pulmonary disease (COPD) in Sweden. A cross-sectional survey study. BMC Med Inform Decis Mak. 2021;21(1):185. doi: 10.1186/s12911-021-01544-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data generated in this study is available upon reasonable request directed to the corresponding author.