Abstract
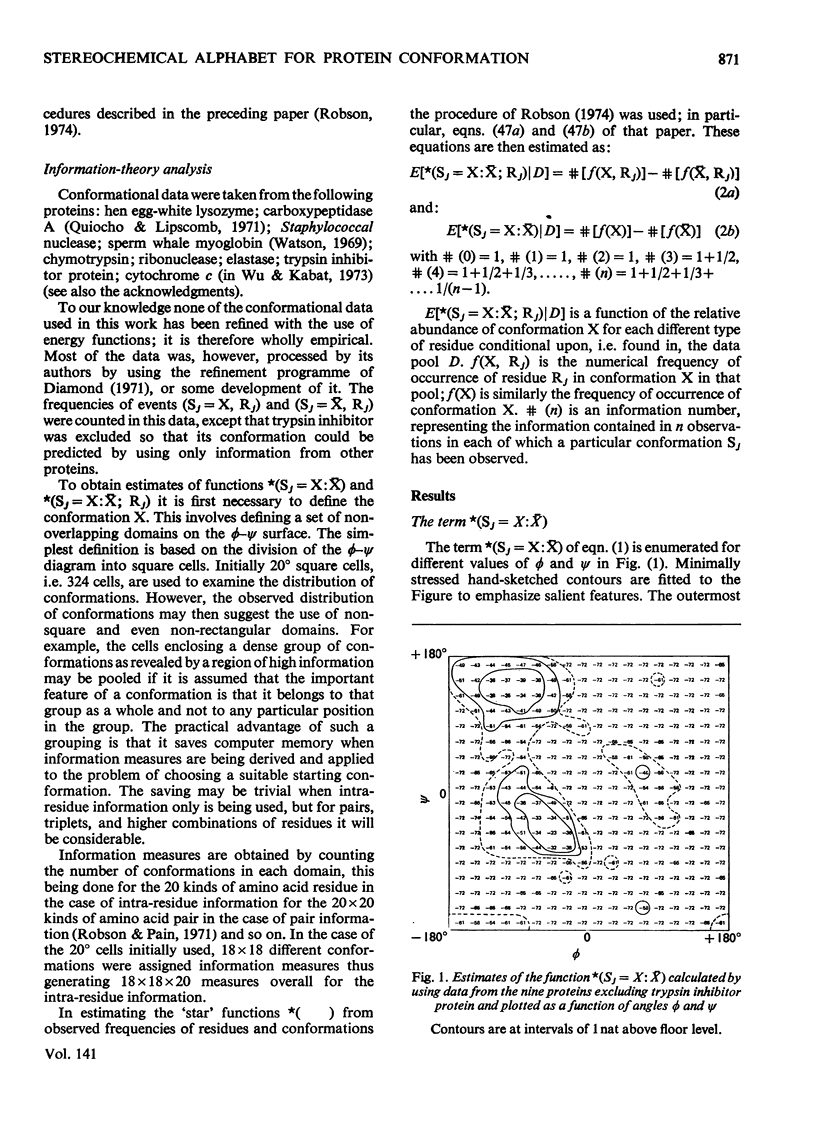
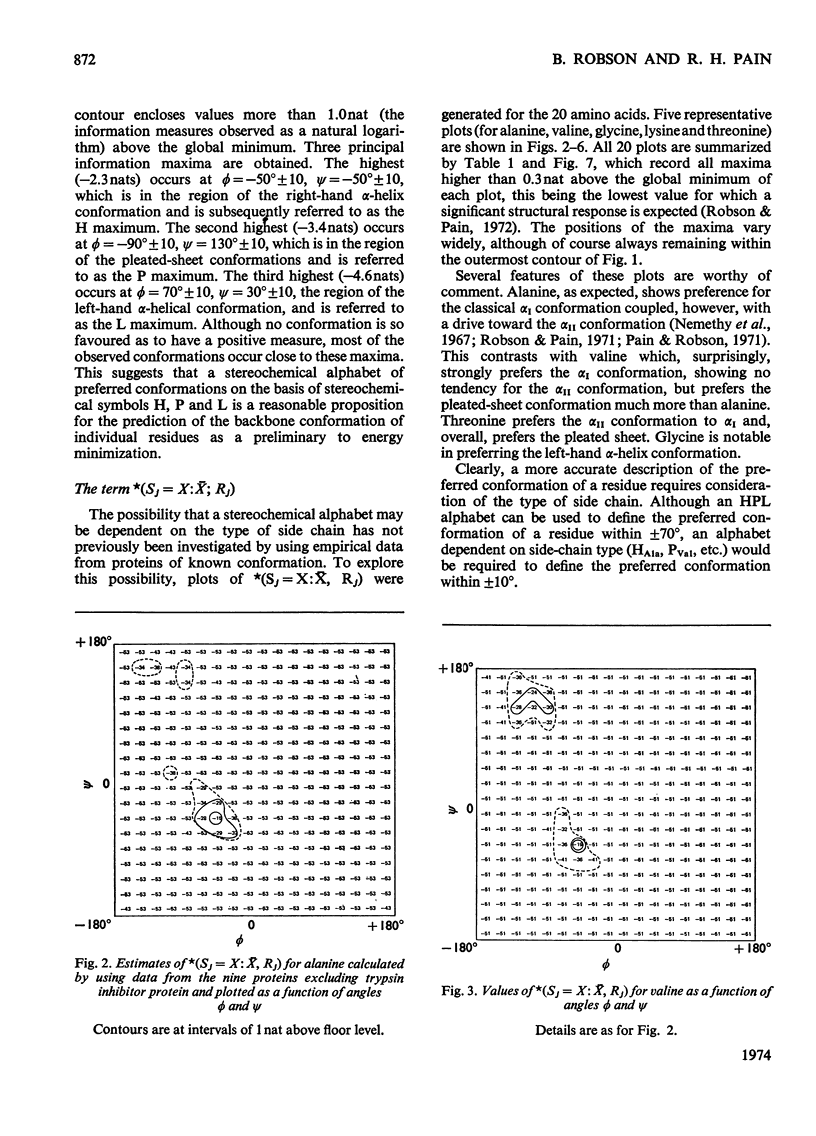
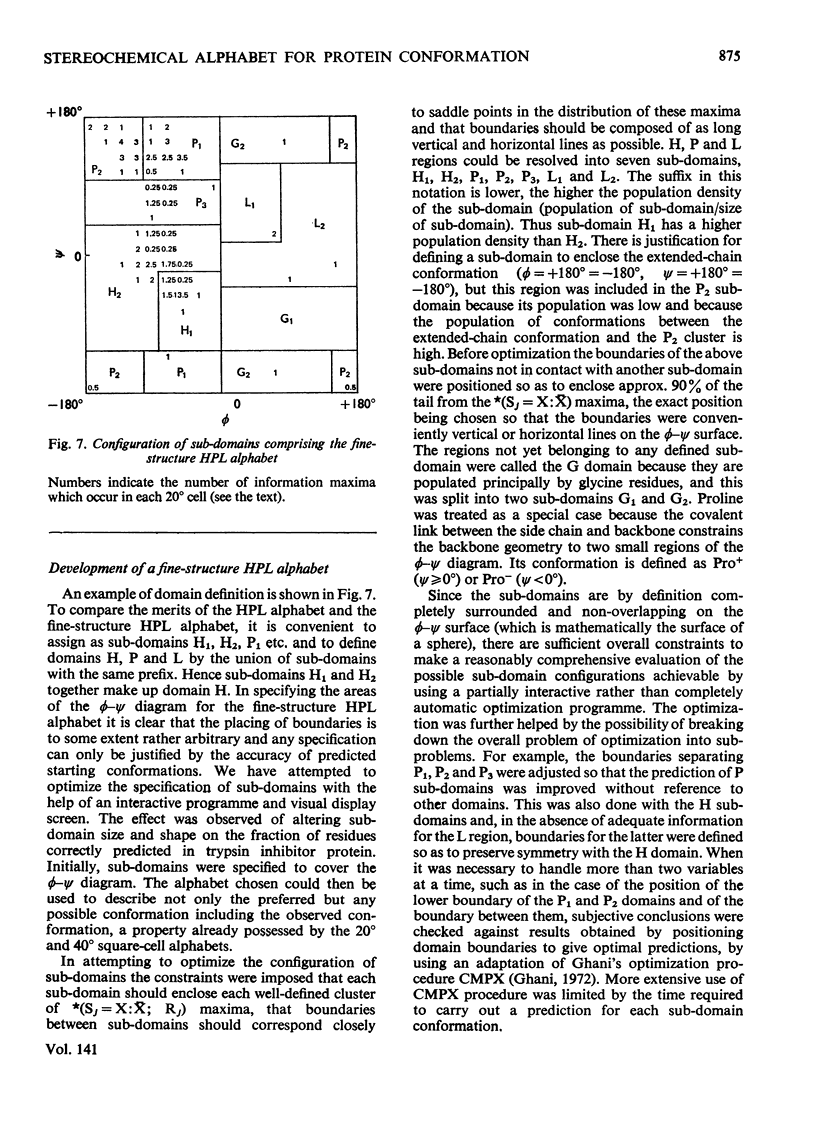
1. The relation of primary sequence to all residue backbone conformations was explored to test out starting conformations for protein folding. 2. Information theory was used to obtain measures of information which quantitate the role of each residue in determining its own conformation; i.e. intra-residue information. 3. The information measures are plotted as a function of ϕ, ψ peptide-backbone angles and ϕ, ψ contour maps obtained for each of the 20 amino acids. These show characteristic differences between residues. 4. To find practical ways of relating sequence to ϕ, ψ angles, several types of stereochemical alphabet were investigated. The value of these was tested by using them to predict the ϕ, ψ angles of nine different proteins. 5. A difference plot was constructed to show regions of the sequence that require little or no information extra to the intra-residue information in order to predict a correct conformation. These regions are suggested to be candidates for nucleating sites in the protein.
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chothia C. Conformation of twisted beta-pleated sheets in proteins. J Mol Biol. 1973 Apr 5;75(2):295–302. doi: 10.1016/0022-2836(73)90022-3. [DOI] [PubMed] [Google Scholar]
- Leach S. J., Némethy G., Scheraga H. A. Computation of the sterically allowed conformations of peptides. Biopolymers. 1966 Apr-May;4(4):369–407. doi: 10.1002/bip.1966.360040402. [DOI] [PubMed] [Google Scholar]
- Lewis P. N., Go N., Go M., Kotelchuck D., Scheraga H. A. Helix probability profiles of denatured proteins and their correlation with native structures. Proc Natl Acad Sci U S A. 1970 Apr;65(4):810–815. doi: 10.1073/pnas.65.4.810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liquori A. M. The stereochemical code and the logic of a protein molecule. Q Rev Biophys. 1969 May;2(1):65–92. doi: 10.1017/s0033583500000792. [DOI] [PubMed] [Google Scholar]
- Némethy G., Phillips D. C., Leach S. J., Scheraga H. A. A second right-handed helical structure with the parameters of the Pauling-Corey alpha-helix. Nature. 1967 Apr 22;214(5086):363–365. doi: 10.1038/214363a0. [DOI] [PubMed] [Google Scholar]
- Pain R. H., Robson B. Analysis of the code relating sequence to secondary structure in proteins. Nature. 1970 Jul 4;227(5253):62–63. doi: 10.1038/227062a0. [DOI] [PubMed] [Google Scholar]
- Pauling L., Corey R. B. Configurations of Polypeptide Chains With Favored Orientations Around Single Bonds: Two New Pleated Sheets. Proc Natl Acad Sci U S A. 1951 Nov;37(11):729–740. doi: 10.1073/pnas.37.11.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Periti P. F., Quagliarotti G., Liquori A. M. Recognition of alpha-helical segments in proteins of known primary structure. J Mol Biol. 1967 Mar 14;24(2):313–322. doi: 10.1016/0022-2836(67)90336-1. [DOI] [PubMed] [Google Scholar]
- Pohl F. M. Empirical protein energy maps. Nat New Biol. 1971 Dec 29;234(52):277–279. doi: 10.1038/newbio234277a0. [DOI] [PubMed] [Google Scholar]
- Quiocho F. A., Lipscomb W. N. Carboxypeptidase A: a protein and an enzyme. Adv Protein Chem. 1971;25:1–78. doi: 10.1016/s0065-3233(08)60278-8. [DOI] [PubMed] [Google Scholar]
- RAMACHANDRAN G. N., RAMAKRISHNAN C., SASISEKHARAN V. Stereochemistry of polypeptide chain configurations. J Mol Biol. 1963 Jul;7:95–99. doi: 10.1016/s0022-2836(63)80023-6. [DOI] [PubMed] [Google Scholar]
- Ramachandran G. N., Sasisekharan V. Conformation of polypeptides and proteins. Adv Protein Chem. 1968;23:283–438. doi: 10.1016/s0065-3233(08)60402-7. [DOI] [PubMed] [Google Scholar]
- Robson B. Analysis of code relating sequences to conformation in globular prtoeins. Theory and application of expected information. Biochem J. 1974 Sep;141(3):853–867. doi: 10.1042/bj1410853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robson B., Pain R. H. Analysis of the code relating sequence to conformation in globular proteins. An informational analysis of the role of the residue in determining the conformation of its neighbours in the primary sequence. Biochem J. 1974 Sep;141(3):883–897. doi: 10.1042/bj1410883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robson B., Pain R. H. Analysis of the code relating sequence to conformation in globular proteins. The distribution of residue pairs in turns and kinks in the backbone chain. Biochem J. 1974 Sep;141(3):899–904. doi: 10.1042/bj1410899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robson B., Pain R. H. Analysis of the code relating sequence to conformation in proteins: possible implications for the mechanism of formation of helical regions. J Mol Biol. 1971 May 28;58(1):237–259. doi: 10.1016/0022-2836(71)90243-9. [DOI] [PubMed] [Google Scholar]
- Robson B., Pain R. H. Directional information transfer in protein helices. Nat New Biol. 1972 Jul 26;238(82):107–108. doi: 10.1038/newbio238107a0. [DOI] [PubMed] [Google Scholar]
- Scheraga H. A. Theoretical and experimental studies of conformations of polypeptides. Chem Rev. 1971 Apr;71(2):195–217. doi: 10.1021/cr60270a003. [DOI] [PubMed] [Google Scholar]
- Schiffer M., Edmundson A. B. Use of helical wheels to represent the structures of proteins and to identify segments with helical potential. Biophys J. 1967 Mar;7(2):121–135. doi: 10.1016/S0006-3495(67)86579-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu T. T., Kabat E. A. An attempt to evaluate the influence of neighboring amino acids (n-1) and (n+1) on the backbone conformation of amino acid (n) in proteins. Use in predicting the three-dimensional structure of the polypeptide backbone of other proteins. J Mol Biol. 1973 Mar 25;75(1):13–31. doi: 10.1016/0022-2836(73)90526-3. [DOI] [PubMed] [Google Scholar]


