Abstract
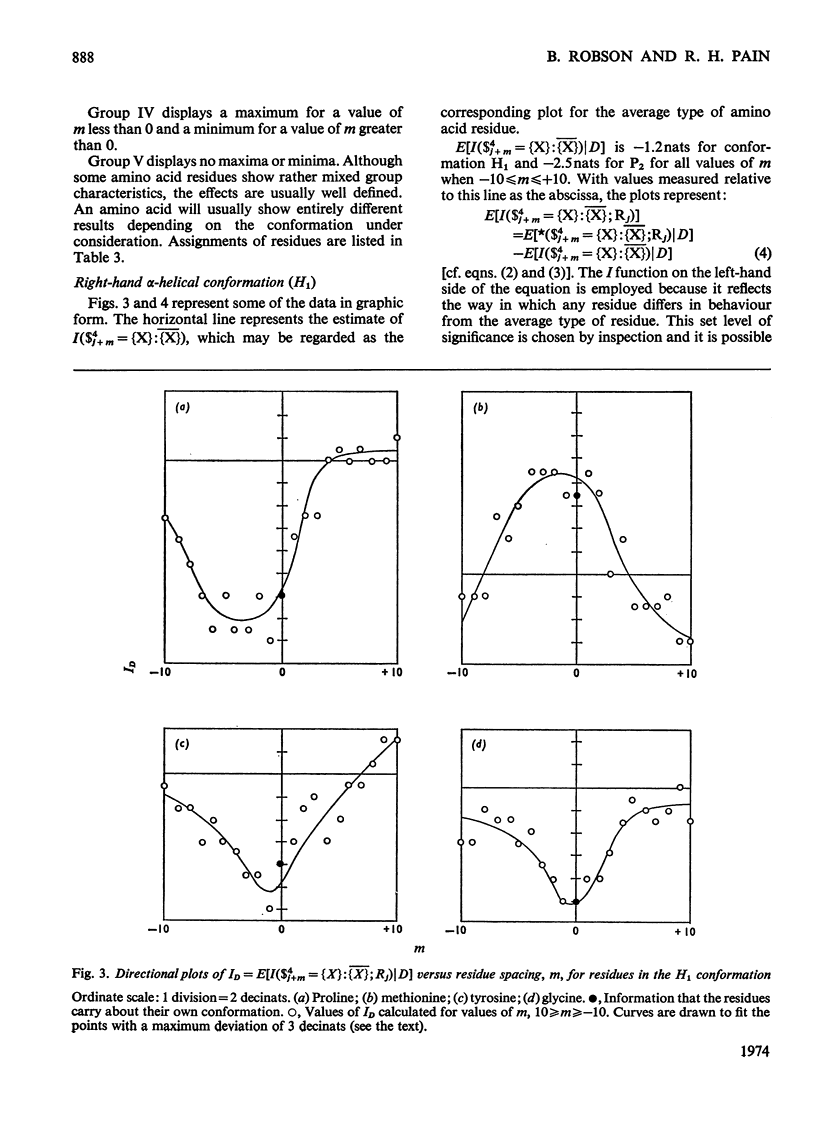
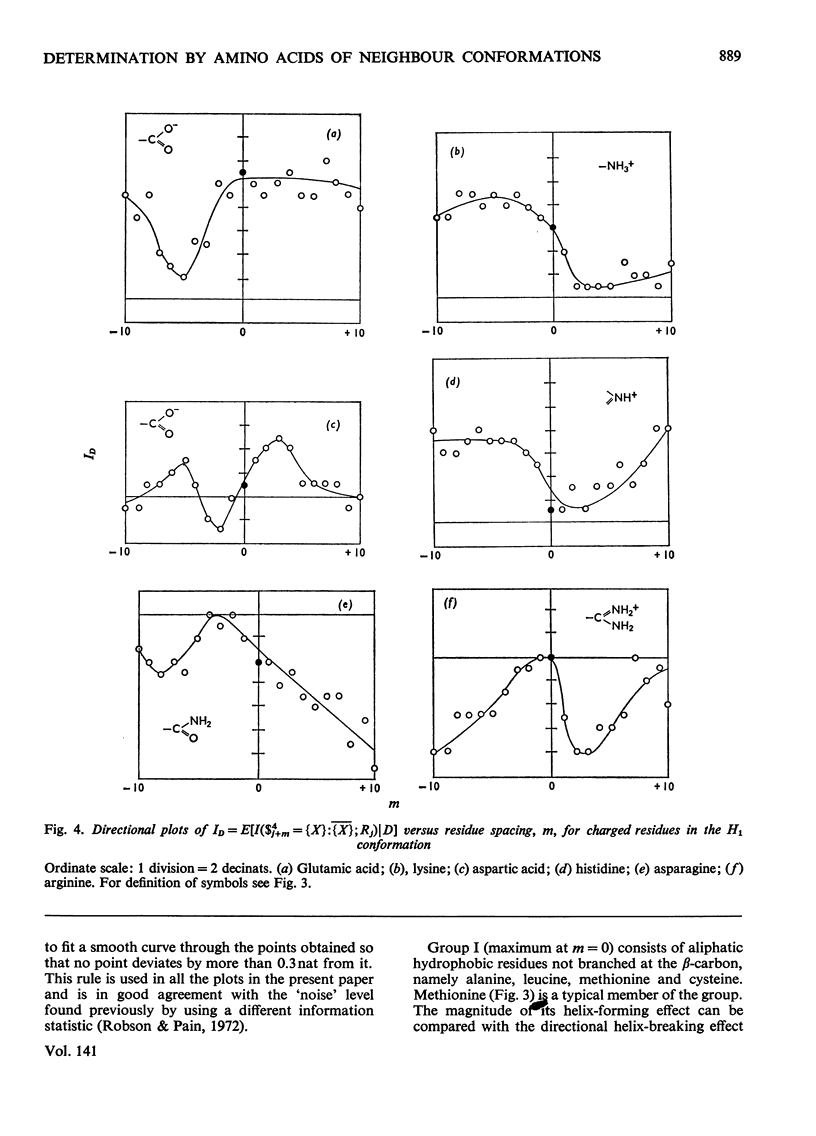
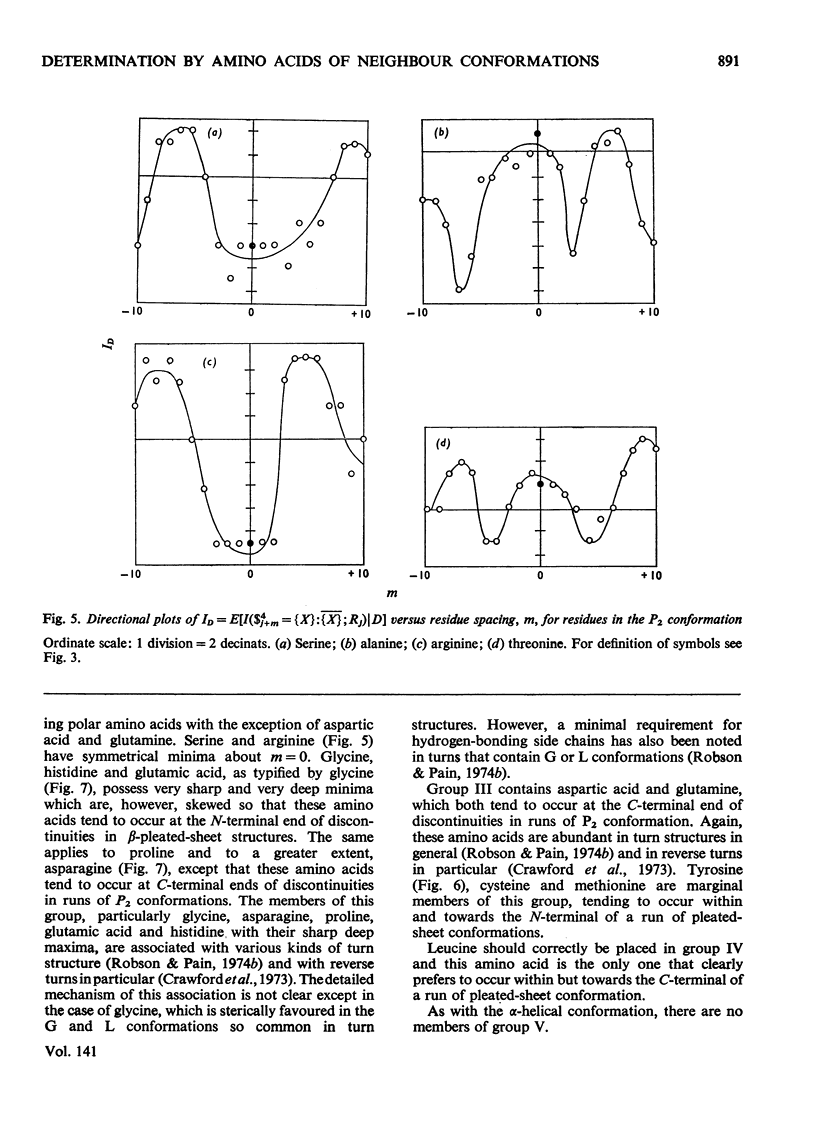
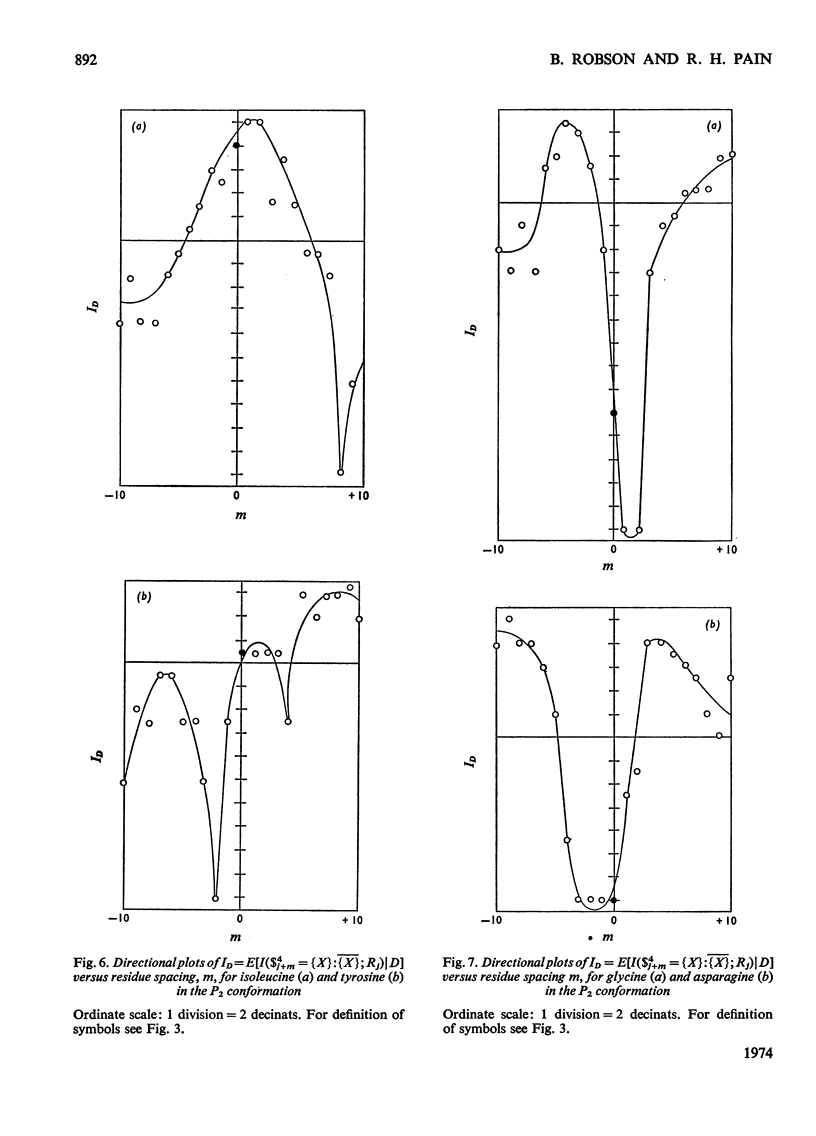
1. The effect exerted by a residue on the conformation of neighbouring residues was analysed by using data from nine globular proteins of known sequence and conformation. 2. An information measure was used which estimated the role of a residue in influencing neighbouring conformations and also its tendency to influence the lengths of runs of residues in that conformation. This measure was estimated for each residue in all conformations defined by domains on the ϕ, ψ diagram. 3. Plots of the information measure yielded an intercept, which was a measure of intra-residue information for a residue. The slope was a measure of the statistical co-operativity or tendency of the residue to influence the occurrence of its neighbours in runs of a particular conformation. Both parameters are a function of the residue type. Statistical co-operativity is found in the α1-helical (H1) and β-pleated-sheet (P2) conformations and, to a lesser extent, in their distorted variants H2 and P1. 4. The directional nature of these influences for H1 and P2 conformations is illustrated by plots of the information measure against the distance m from the residue, for m=−10 to +10. 5. The results for statistical co-operativity are discussed in relation to theories of helix–coil and pleated-sheet–coil transitions. The value of the information-theory-derived parameters in obtaining s parameters for the Zimm & Bragg (1959) equations is illustrated. 6. Directional effects are discussed with particular relation to mechanisms of the termination of helices and the involvement of the αII conformation and also to discontinuities in pleated-sheet conformations.
Full text
PDF




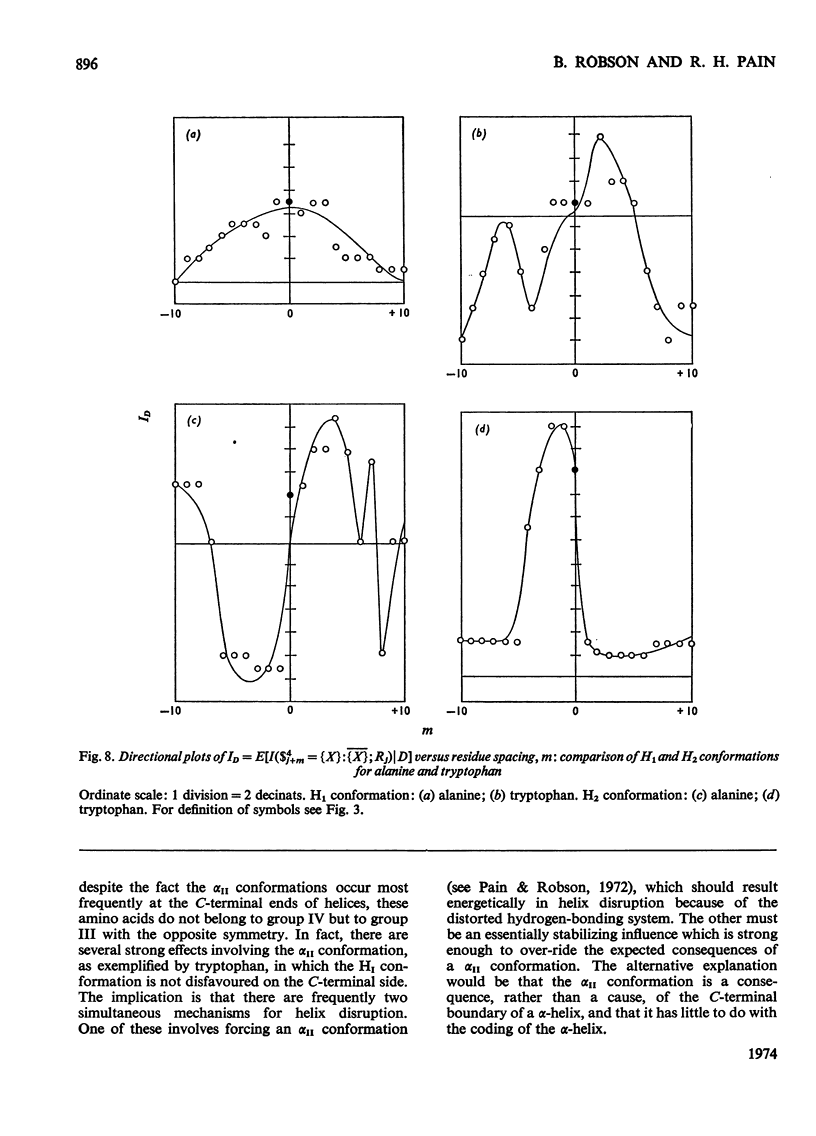

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anfinsen C. B. The formation of the tertiary structure of proteins. Harvey Lect. 1967;61:95–116. [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Conformational parameters for amino acids in helical, beta-sheet, and random coil regions calculated from proteins. Biochemistry. 1974 Jan 15;13(2):211–222. doi: 10.1021/bi00699a001. [DOI] [PubMed] [Google Scholar]
- Crawford J. L., Lipscomb W. N., Schellman C. G. The reverse turn as a polypeptide conformation in globular proteins. Proc Natl Acad Sci U S A. 1973 Feb;70(2):538–542. doi: 10.1073/pnas.70.2.538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzzo A. V. The influence of amino-acid sequence on protein structure. Biophys J. 1965 Nov;5(6):809–822. doi: 10.1016/S0006-3495(65)86753-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havsteen B. H. A study of the correlation between the amino acid composition and the helical content of proteins. J Theor Biol. 1966 Jan;10(1):1–10. doi: 10.1016/0022-5193(66)90174-3. [DOI] [PubMed] [Google Scholar]
- Kotelchuck D., Scheraga H. A. The influence of short-range interactions on protein conformation. I. Side chain-backbone interactions within a single peptide unit. Proc Natl Acad Sci U S A. 1968 Dec;61(4):1163–1170. doi: 10.1073/pnas.61.4.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotelchuck D., Scheraga H. A. The influence of short-range interactions on protein onformation. II. A model for predicting the alpha-helical regions of proteins. Proc Natl Acad Sci U S A. 1969 Jan;62(1):14–21. doi: 10.1073/pnas.62.1.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis P. N., Scheraga H. A. Predictions of structural homologies in cytochrome c proteins. Arch Biochem Biophys. 1971 Jun;144(2):576–583. doi: 10.1016/0003-9861(71)90363-8. [DOI] [PubMed] [Google Scholar]
- Némethy G., Phillips D. C., Leach S. J., Scheraga H. A. A second right-handed helical structure with the parameters of the Pauling-Corey alpha-helix. Nature. 1967 Apr 22;214(5086):363–365. doi: 10.1038/214363a0. [DOI] [PubMed] [Google Scholar]
- Pain R. H., Robson B. Analysis of the code relating sequence to secondary structure in proteins. Nature. 1970 Jul 4;227(5253):62–63. doi: 10.1038/227062a0. [DOI] [PubMed] [Google Scholar]
- Prothero J. W. Correlation between the distribution of amino acids and alpha helices. Biophys J. 1966 May;6(3):367–370. doi: 10.1016/S0006-3495(66)86662-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ptitsyn O. B. Statistical analysis of the distribution of amino acid residues among helical and non-helical regions in globular proteins. J Mol Biol. 1969 Jun 28;42(3):501–510. doi: 10.1016/0022-2836(69)90238-1. [DOI] [PubMed] [Google Scholar]
- Quiocho F. A., Lipscomb W. N. Carboxypeptidase A: a protein and an enzyme. Adv Protein Chem. 1971;25:1–78. doi: 10.1016/s0065-3233(08)60278-8. [DOI] [PubMed] [Google Scholar]
- Robson B. Analysis of code relating sequences to conformation in globular prtoeins. Theory and application of expected information. Biochem J. 1974 Sep;141(3):853–867. doi: 10.1042/bj1410853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robson B., Pain R. H. Analysis of the code relating sequence to conformation in globular proteins. Development of a stereochemical alphabet on the basis of intra-residue information. Biochem J. 1974 Sep;141(3):869–882. doi: 10.1042/bj1410869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robson B., Pain R. H. Analysis of the code relating sequence to conformation in globular proteins. The distribution of residue pairs in turns and kinks in the backbone chain. Biochem J. 1974 Sep;141(3):899–904. doi: 10.1042/bj1410899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robson B., Pain R. H. Analysis of the code relating sequence to conformation in proteins: possible implications for the mechanism of formation of helical regions. J Mol Biol. 1971 May 28;58(1):237–259. doi: 10.1016/0022-2836(71)90243-9. [DOI] [PubMed] [Google Scholar]
- Robson B., Pain R. H. Directional information transfer in protein helices. Nat New Biol. 1972 Jul 26;238(82):107–108. doi: 10.1038/newbio238107a0. [DOI] [PubMed] [Google Scholar]
- Tanford C. Protein denaturation. Adv Protein Chem. 1968;23:121–282. doi: 10.1016/s0065-3233(08)60401-5. [DOI] [PubMed] [Google Scholar]


