Abstract
Abstract
Campylobacter jejuni, a major cause of foodborne zoonotic infections worldwide, shows a paradoxical ability to survive despite its susceptibility to environmental and food-processing stressors. This resilience is likely due to the bacterium entering a viable but non-culturable state, often within biofilms, or even initiating biofilm formation as a survival strategy. This study presents an innovative application of NanoLuc bioluminescence to accurately monitor the development of C. jejuni biofilms on various substrates, such as polystyrene plates, mucin-coated surfaces, and chicken juice matrices. Introduction of NanoLuc luciferase in a pathogenic C. jejuni strain enables rapid non-invasive holistic observation, capturing a spectrum of cell states that may comprise live, damaged, and viable but non-culturable populations. Our comparative analysis with established biofilm quantification methods highlights the specificity, sensitivity, and simplicity of the NanoLuc assay. The assay is efficient and offers precise cell quantification and thus represents an important complementary or alternative method to conventional biofilm monitoring methods. The findings of this study highlight the need for a versatile approach and suggest combining the NanoLuc assay with other methods to gain comprehensive insight into biofilm dynamics.
Key points
• Innovative NanoLuc bioluminescence assay for sophisticated biofilm quantification.
• Holistic monitoring of C. jejuni biofilm by capturing live, damaged and VBNC cells.
• Potential for improving understanding of biofilm development and structure.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00253-024-13383-0.
Keywords: Campylobacter jejuni, Biofilm quantification, NanoLuc bioluminescence, VBNC, Food safety, Foodborne infections
Introduction
Compared to other foodborne pathogens, Campylobacter jejuni is particularly susceptible to numerous environmental and food-processing stress factors, such as fluctuations in osmotic pressure, temperature, and pH. However, the bacterium remains the most frequently reported cause of foodborne zoonotic infection (EFSA 2023). C. jejuni is found in the gastrointestinal tract of many domestic and wild birds and mammals, and consumption of undercooked poultry, raw milk, and contaminated products is strongly associated with C. jejuni infection (Hermans et al. 2012; Ijaz et al. 2018). Although C. jejuni is traditionally thought to be unable to survive outside the host in natural aerobic environments or in the food chain (Solomon and Hoover 1999; Klančnik et al. 2014; Giaouris et al. 2015), recent evidence suggests that it is unexpectedly widespread in the environment and can be easily detected in food, water, and other sources (Good et al. 2019; Tram et al. 2020).
The persistence of Campylobacter may be related to its ability to form biofilms. Biofilm formation is influenced by various stress response mechanisms, including changes in cell morphology, hydrophobicity, membrane proteins, quorum sensing, stress responses, and adhesion. Campylobacter can adapt to stress conditions by transitioning to a viable but non-culturable state (VBNC) while maintaining its membrane integrity and metabolic activity (Ramamurthy et al. 2014). Biofilms can be both accelerated and induced by the transition to the VBNC state. Microorganisms within biofilms are encased within a matrix of extracellular polymeric substances that acts as a protective barrier against potentially hostile conditions. These abilities underline a critical aspect of food safety and may explain the persistence of Campylobacteriosis in developed countries despite strict hygiene standards. However, survival strategies of biofilms remain poorly understood and are not adequately addressed by existing methodology. This emphasizes the importance of understanding these specific conditions for developing effective prevention of Campylobacteriosis (Tram et al. 2020; Teh et al. 2014; Jackson et al. 2009; Klančnik et al. 2009).
It is crucial to select relevant, reliable, robust, and consistent methods that identify adhesion or biofilm formation under different environmental conditions. Conventional cultivation methods, in which the colony count is determined, can be complemented by crystal violet staining of adhered or biofilm cells (Trošt et al. 2016; Duarte et al. 2015; Kurinčič et al. 2016; Klančnik et al. 2017a). In addition, metabolic activity in biofilms is assessed colorimetrically by tracking the conversion of tetrazolium salts to formazan (Ma et al. 2022; Wagle et al. 2019; Duarte et al. 2015) while simultaneously measuring the presence of ATP by bioluminescence with BacTiter-Glo or resazurin fluorescence (Trošt et al. 2016; Klančnik et al. 2021). Refined methods have advanced the study of biofilms, focusing on their physical properties and heterogeneity. These include fluorescent staining or immunofluorescence in conjunction with flow cytometry or fluorescence microscopy. For example, SYTO-9 and propidium iodide staining provides insights into cell localization and biofilm heterogeneity (Oh et al. 2018; Wagle et al. 2019; Ramić et al. 2021). In addition, various dyes are used to characterize individual extracellular polymeric substances of biofilms, thereby elucidating biofilm composition and function (Azeredo et al. 2017). Furthermore, molecular (e.g., PCR-based) techniques have been used to determine the presence of C. jejuni in both mono- and multi-species cultures, providing insights into the genetic landscape of cell communities (Winkelströter et al. 2014; Klančnik et al. 2017b). To elucidate the structure, morphology, and bacterial interactions within biofilms, advanced microscopic techniques, such as transmission and scanning electron microscopy, are used (Azeredo et al. 2017; Wagle et al. 2019).
Given the multi-layered nature of biofilms, finding methods that capture this complexity is challenging. This need is underlined by the limitations of current methods, which are generally expensive, time-consuming, non-specific, technically complicated, and unsuitable for accurate, high-throughput biofilm cell quantification (Franklin et al. 2015; Alves et al. 2020; Carrascosa et al. 2021).
Our aim was to assess C. jejuni biofilm formation on microtiter plates using NanoLuc luciferase from Oplophorus gracilirostris for 4, 8, 24, 48, and 72 h. This method benefits from a luminescent response that does not depend on the presence of ATP and minimizes background noise (England et al. 2016). Our approach involves holistic monitoring of C. jejuni biofilm by capturing live, damaged, and VBNC cells in different environments, from abiotic surfaces (e.g., polystyrene plates) to biotic mucin-coated variants and food matrices (e.g., chicken juice). We also investigated the biofilm dynamics in multispecies biofilm with a Salmonella strain. For this purpose, we developed a C. jejuni strain expressing NanoLuc, according to our previous work with non-pathogenic Listeria innocua (Berlec et al. 2021). NanoLuc luciferase is characterized by its compact size (19 kDa), thermal stability (Tm = 60 °C), efficacy in a pH range of 6–8, lack of post-translational modifications or disulfide bonds, consistent cellular distribution, and efficient bioluminescence (England et al. 2016).
Genetic manipulation of Campylobacter is a particular challenge, as protocols that are effective in other bacteria often fail in Campylobacter (Davis et al. 2008). The NanoLuc protein, due to its small size, facilitates genetic manipulation and improves protein expression, making it a more viable option in such difficult systems. For this reason, it was chosen over traditional bacterial luciferases Fluc and Rluc (Syed and Anderson 2021). Its superior signal amplification and increased sensitivity also make NanoLuc a more rational choice compared to fluorescence proteins.
Material and methods
Bacterial strains and culture conditions
C. jejuni 81–176 that was originally isolated from humans (deposited as ATCC BAA-2151™; GenBank accession number NC_008787.1) was cultured at 42 °C in Mueller–Hinton (MH) broth (BD Difco, Fischer Scientific, NH) under microaerophilic conditions (85% N2, 10% CO2, and 5% O2), either in liquid form with gentle shaking (80 rpm) or in the same medium solidified with 1.7% agar. Escherichia coli DH5α (New England Biolabs, MA) and Salmonella enterica from a retail poultry meat sample were cultured at 37 °C with aeration in either lysogeny broth (BD Difco, Fischer Scientific, NH) or MH broth, respectively.
Molecular cloning
The NanoLuc gene was back-translated from the NanoLuc protein (Hall et al. 2012) and codon-optimized for C. jejuni (GenBank accession number OR958835). It was synthesized in fusion with the promoter porA (Elgamoudi and Ketley 2018) at the 5’-end and a transcription terminator at the 3’-end (gBlock, Integrated DNA Technologies, Leuven, Belgium; Supplementary Figure S1). Restriction recognition sites were added by PCR amplification, with the primers PorAnLuc-Bam-F (5′-ATTTGGATCCTTTAAAACAACTATATATTAC-3′) and Harp-nLuc-Xba-R (5′-ATTTTCTAGATGGCAGTTTATGGCGGGCGTCCTGCCCGCCACCCTCCGGGCCGTTGCTTCGCAACGTTCAAATCCGCTCCCG-3′). The PCR amplicon was cloned into the pMW10 plasmid (Wösten et al. 1998) using the restriction enzymes BamHI and XbaI (Fast Digest, Thermo Scientific, Vilnius, Lithuania) to obtain the plasmid pMW10_nLuc (Supplementary Figure S1) and transformed into E. coli with heat shock. The plasmid DNA was isolated using the NucleoSpin Plasmid PCR kit (Macherey–Nagel, Dueren, Germany) and subsequently sequenced (Eurofins Genomics, Konstanz, Germany). Transformation of C. jejuni was performed by triparental mating as described previously (Miller et al. 2000).
NanoLuc bioluminescence assay
The method was adapted according to the protocol of Berlec et al. (2022). Bioluminescence was measured using a plate reader (in luminescence mode; BMG Labtech, Ortenberg, Germany) in white flat-bottom 96-well plates (Thermo Scientific, Waltham, MA). The Nano-Glo Luciferase Assay System (Promega, Madison, WI) was prepared according to the manufacturer’s instructions. Bacterial samples (50 µL) were mixed with reagent (50 µL) and incubated at room temperature for 10 min prior to measurements.
Construction of a calibration curve was performed using bacterial dispersions in phosphate-buffered saline (PBS) with different cell densities (1.50 × 108, 1.13 × 108, 7.50 × 107, 3.75 × 107, and 1.88 × 107 colony-forming units (CFU)/mL). Those were prepared in duplicate from the overnight cultures. Using the generated calibration curve, the concentrations of C. jejuni in the samples were determined. The limit of detection (LOD) and limit of quantification (LOQ), accuracy, and precision were calculated as in Berlec et al. (2021). Accuracy and precision were determined using known concentrations of C. jejuni (i.e., 1.2 × 108, 7.0 × 107, and 2.5 × 107 CFU/mL). The parameters were determined on three separate days, to assess the repeatability of the test.
Assays for the detection and quantification of C. jejuni biofilms on polystyrene surfaces
Initial cultures for biofilm assays were prepared with different concentrations of C. jejuni (2.5 × 107, 6.0 × 107, 1.0 × 108, and 3.0 × 108 CFU/mL) by dilution. They were transferred (200 µl) to white flat-bottomed 96-well plates with lids (Thermo Scientific) for bioluminescence measurement or clear flat-bottomed 96-well plates with lids (Corning, Corning, NY) for plate counting (Berlec et al. 2021), crystal violet (Kurinčič et al. 2016), and resazurin (Kovač et al. 2015) assays. Plates for bioluminescence measurement and plate counting were incubated at 42 °C without shaking for different periods of time (4, 8, 24, 48, and 72 h). Both the crystal violet and resazurin assays were first performed 24 h after inoculation, as the preliminary analysis after shorter incubation times resulted in signals below the minimum reliable signal due to the low number of bacteria.
The data obtained with the different biofilm quantification methods were analyzed, and the relationships between the quantified cells and incubation times at different initial inoculum concentrations were investigated. Using linear regression for each method, the slope values were standardized based on the average value for each method to provide an understanding of the underlying data trends.
Quantification of biofilm C. jejuni NanoLuc cells on mucin-coated surfaces
The bioluminescence assay for C. jejuni cell quantification was also applied to mucin-coated microtiter plates, which were prepared according to Jug et. al (2024). One day before the planned cell inoculation, dilutions of the mucin solutions were prepared at concentrations of 500 µg/mL and 50 µg/mL. The initial inoculum concentration was 1.22 × 108 CFU/mL in all samples. Cells were quantified by measuring bioluminescence and assessing cultivability as described above.
Biofilm quantification of C. jejuni NanoLuc inoculated in a food model and in mixed culture with S. enterica
A food model of chicken meat juice (Piskernik et al. 2011) was used as medium for biofilm formation. Initial inocula of C. jejuni with a cell density of 1 × 108 CFU/mL (OD600 = 0.2) were prepared in chicken juice and MH broth (as a control). The inocula were pipetted onto a white or clear flat-bottomed microtiter plate, which were incubated for 24–48 h at 42 °C in a microaerophilic atmosphere with six biological replicates for chicken juice and three for the control; three technical replicates were performed for all biological replicates.
To quantify C. jejuni in mixed culture with S. enterica, separate inocula were prepared in MH broth for both species. The cell densities were measured, and initial inocula with a cell density of 1 × 108 CFU/mL (OD600 = 0.2) were prepared. Both inocula (100 μL each) were pipetted into a single well of a white or clear flat-bottomed microtiter plate and mixed thoroughly with a pipette. This procedure was repeated for three biological replicates, each in three technical replicates. The plates containing the mixed cultures were then incubated at 42 °C in a microaerophilic atmosphere for 24–48 h. Cell density was determined by measuring bioluminescence and by assessing cultivability on MH agar plates with added 30 µg/mL kanamycin to ensure C. jejuni selection.
Statistical analysis
Statistical analyses were performed using GraphPad Prism (GraphPad Software, San Diego, USA) and R (R Foundation for Statistical Computing, Vienna, Austria). In GraphPad Prism, the relationship between the quantified cells and the incubation time at different initial inoculum concentrations was observed. The slope values were standardized based on the average value for each method. In addition, ANOVA analysis was performed in R to test whether significant changes occurred as a function of initial inoculum concentration or incubation time.
Results
Heterologous expression and functionality of NanoLuc in C. jejuni
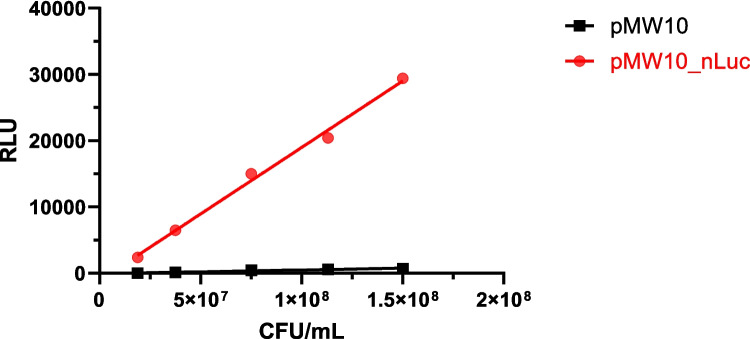
To confirm the heterologous expression of NanoLuc in C. jejuni, bioluminescence was compared between C. jejuni with the NanoLuc gene (plasmid pMW10_nLuc) and with the empty plasmid (pMW10). NanoLuc-expressing C. jejuni showed a significantly higher and cell-density-dependent bioluminescence intensity (Fig. 1).
Fig. 1.
Bioluminescence intensity represented as relative light units (RLU) as a function of concentration (CFU/mL) of C. jejuni expressing NanoLuc (i.e., containing the plasmid pMW10_nLuc; red) or control C. jejuni (i.e., containing the empty plasmid pMW10; black)
NanoLuc bioluminescence assay parameters
Limit of detection, limit of quantification, accuracy, and precision were assessed in the NanoLuc bioluminescence assay. Repeatability was evaluated by performing the experiment on different days (E1, E2, E3). Calibration curves showed good repeatability and consistently achieved R2 values of > 0.98 in separate experiments (Supplementary Figure S2). The mean values of the limit of detection and limit of quantification from three separate experiments were 9.12 (± 2.13) × 106 and 1.61 (± 0.48) × 107 CFU/mL, respectively. The accuracy of the NanoLuc bioluminescence assay was determined for three different C. jejuni concentrations (1.20 × 108, 7.00 × 107, and 2.50 × 107 CFU/mL). Each concentration was assessed on three different days in two independent biological replicates and three technical replicates (Table 1). Most samples had a relative error of < 30%, with two samples exceeding 30%, which we consider acceptable (Berlec et al. 2021). Precision was assessed by testing the same three C. jejuni concentrations in six technical replicates. To determine repeatability, precision was also determined on three different days. The coefficients of variation were below 20% (mostly below 9%) for all samples, indicating high precision and good repeatability of the NanoLuc bioluminescence assay (Table 2).
Table 1.
Accuracy of the NanoLuc bioluminescence assays on three separate days (E1, E2, E3). Three different C. jejuni concentrations were determined by bioluminescence measurement, each as two technical repeats. Data are presented as mean (± standard error). CFU colony-forming unit
| Concentration (CFU/mL) | Determined concentration (CFU/mL) | Relative error (%) | ||||
|---|---|---|---|---|---|---|
| E1 | E2 | E3 | E1 | E2 | E3 | |
| 1.20 × 108 | 1.05 (± 0.06) × 108 | 1.20 (± 0.07) × 108 | 1.15 (± 0.02) × 108 | 12.30 | 0.06 | 3.89 |
| 1.20 × 108 | 9.39 (± 0.24) × 107 | 8.49 (± 0.83) × 107 | 1.44 (± 0.06) × 108 | 21.73 | 29.24 | 20.01 |
| 7.00 × 107 | 6.79 (± 0.63) × 107 | 8.61 (± 0.17) × 107 | 8.21 (± 0.54) × 107 | 3.01 | 22.94 | 17.28 |
| 7.00 × 107 | 6.32 (± 0.47) × 107 | 6.70 (± 0.58) × 107 | 9.88 (± 0.06) × 107 | 9.68 | 4.29 | 41.12 |
| 2.50 × 107 | 3.20 (± 0.19) × 107 | 2.73 (± 0.17) × 107 | 3.27 (± 0.13) × 107 | 28.07 | 9.24 | 30.66 |
| 2.50 × 107 | 2.47 (± 0.16) × 107 | 2.15 (± 0.11) × 107 | 2.78 (± 0.17) × 107 | 1.10 | 14.18 | 11.26 |
Table 2.
Precision of the NanoLuc bioluminescence assays on three separate days (E1, E2, E3). Three concentrations were determined by bioluminescence measurement. Data are presented as mean (± standard error). CFU colony-forming unit
| Concentration (CFU/mL) | Determined concentration (CFU/mL) | Coefficient of variation (%) | ||||
|---|---|---|---|---|---|---|
| E1 | E2 | E3 | E1 | E2 | E3 | |
| 1.20 × 108 | 1.81 (± 0.06) × 108 | 1.27 (± 0.04) × 108 | 1.16 (± 0.01) × 108 | 7.47 | 8.48 | 2.04 |
| 7.00 × 107 | 6.93 (± 0.29) × 107 | 8.94 (± 0.28) × 107 | 8.11 (± 0.28) × 107 | 10.32 | 7.69 | 8.51 |
| 2.50 × 107 | 2.81 (± 0.21) × 107 | 2.51 (± 0.19) × 107 | 3.49 (± 0.12) × 107 | 18.05 | 18.98 | 8.24 |
NanoLuc bioluminescence assay for monitoring C. jejuni biofilm for up to 72 h in comparison to established methods
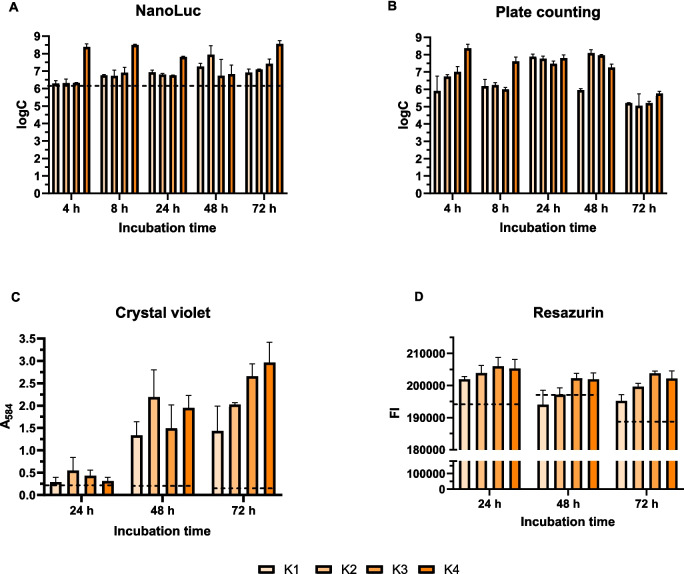
C. jejuni biofilm formation on polystyrene microtiter plates was initiated with different inoculum concentrations. Biofilm formation was monitored for 72 h using various methods, including the NanoLuc bioluminescence assay, plate counting of culturable cells, crystal violet biomass staining, and resazurin metabolic activity staining (Fig. 2). NanoLuc bioluminescence assay revealed that biofilm formation depended on inoculum concentration. Bacterial cell concentrations at each time point were calculated from calibration curves and were all above the limit of detection.
Fig. 2.
Monitoring biofilm formation on polystyrene plates over 72 h using the NanoLuc bioluminescence assay (A), plate counting assay (B), crystal violet staining (C), and resazurin fluorescence (D). Initial inocula concentrations of 2.50 × 107, 6.00 × 107, 1.00× 108, and 3.00 × 108 CFU/mL are denoted as K1, K2, K3, and K4, respectively. Each cultivation was performed in three biological and three technical replicates. Error bars denote standard deviation, and the horizontal dashed black lines denote the limit of detection (NanoLuc) or minimal reliable signal (crystal violet and resazurin). logC, logarithm of CFU/mL; FI, fluorescence intensity; A584, absorbance at 584 nm
The plate counting method revealed that the number of cells increased for most inoculum concentrations after 24 and 48 h compared to 4 h of incubation. However, after 72 h of incubation, a decrease in CFU/mL was observed regardless of the concentration of the initial inoculum (Fig. 2B). Compared with the plate counting method, the bioluminescence method revealed smaller differences in the numbers of cells after different incubation times (Fig. 2A). The decrease in cell viability after 72 h of incubation was not observed with the luminescence method. Crystal violet staining revealed increased absorbance with incubation time for all four concentrations of the initial inoculum (Fig. 2C). By contrast, resazurin staining (Fig. 2D) revealed a decrease in the measured values after 48 and 72 h of incubation.
Analysis of the data obtained revealed a pattern in the biofilm quantification results, depending on the methods used. A positive slope value trend was observed with the use of NanoLuc and crystal violet staining, whereas a negative slope value trend was observed with the plate counting method and resazurin fluorescence assay (Supplementary Figure S3). The ANOVA analysis showed that the incubation time of the biofilm has a statistically significant influence on the change in absorption for crystal violet (p = 0.003735). For resazurin, the incubation time also had a statistically significant effect (p = 0.02325), while the concentration was just above the significance threshold (p = 0.05002) (Supplementary Table S1).
Monitoring C. jejuni NanoLuc biofilm on mucin-coated microtiter plates
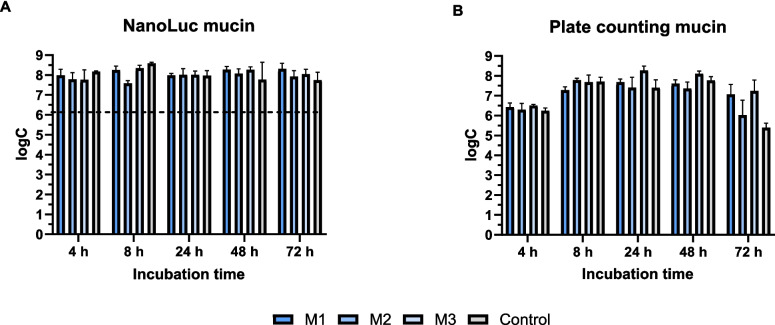
Three different mucin concentrations were used to monitor C. jejuni biofilm on mucin-coated microtiter plates. The control represented biofilm growth on polystyrene surfaces without mucin coating (Fig. 3). The numbers of quantified cells did not significantly differ between the different mucin concentrations. The plate counting method showed the highest number of cells at the lowest mucin concentration (50 µg/mL) after 24 and 48 h of incubation. After 72 h, the numbers of live cells decreased, with the greatest decrease on the uncoated surface. In contrast to the plate counting method, the bioluminescence method did not reveal any decrease in cell numbers after 72 h of incubation.
Fig. 3.
Monitoring biofilm formation on mucin-coated polystyrene plates over 72 h using the NanoLuc bioluminescence assay (A) and plate counting assay (B). Mucin was applied at concentrations of 1 mg/mL (M1), 500 µg/mL (M2), and 50 µg/mL (M3). Each cultivation was performed in three biological and three technical replicates. Error bars denote standard deviation, and the horizontal dashed black lines denote the limit of detection (NanoLuc). logC, logarithm of CFU/mL
Quantification of C. jejuni NanoLuc biofilm in chicken juice and in mixed culture with S. enterica
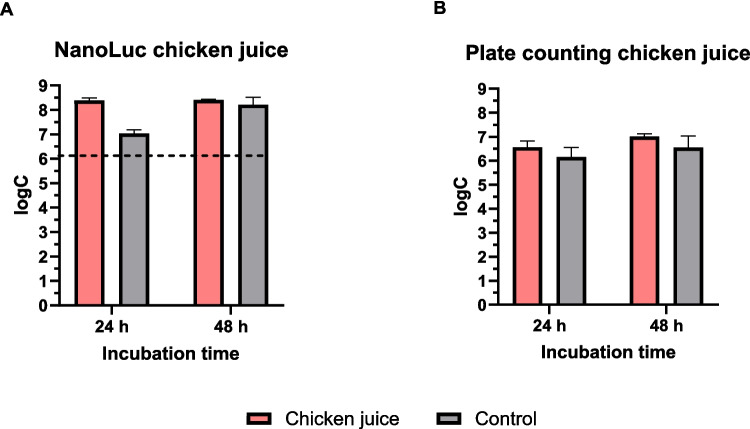
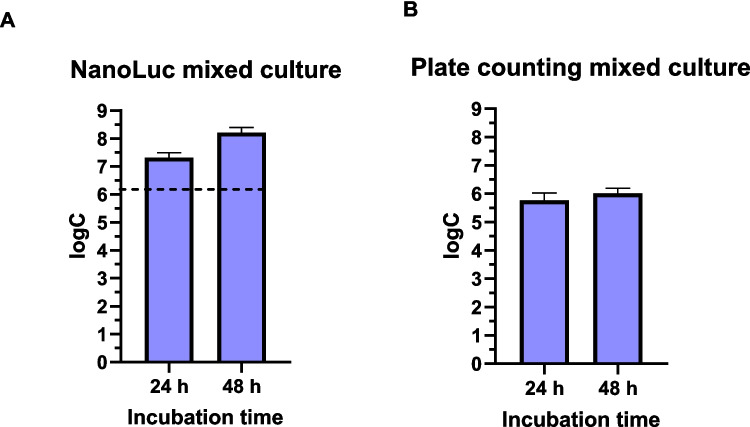
The formation of C. jejuni NanoLuc biofilm in chicken juice was quantified and compared to that in MH broth. Bioluminescence quantification revealed similar cell numbers after 24 and 48 h of incubation, whereas the plate counting method revealed a lower cell number after 24 h compared with 48 h of incubation (Fig. 4). Also, the concentration of C. jejuni NanoLuc cells in mixed biofilm with S. enterica was quantified (Fig. 5). Compared to plate counting, bioluminescence measurements revealed higher number of cells after 24 and 48 h of incubation.
Fig. 4.
Monitoring biofilm formation in chicken juice over 48 h using the NanoLuc bioluminescence assay (A) and plate counting assay (B). Mueller–Hinton broth was used as a control. Error bars represent standard deviation of six biological replicates in three technical replicates (chicken juice) or three biological replicates in three technical replicates (controls). The horizontal dashed black line denotes the limit of detection (NanoLuc). logC, logarithm of CFU/mL
Fig. 5.
Monitoring Campylobacter jejuni quantity during biofilm formation in a mixed culture with Salmonella enterica over 48 h using the NanoLuc bioluminescence assay (A) and the plate counting assay (B). Error bars represent standard error of six biological replicates and three technical replicates, and the horizontal dashed black lines denote the limit of detection (NanoLuc). logC, logarithm of CFU/mL
Discussion
We underline the urgent need for increased surveillance and measures to contain foodborne bacterial infections, as emphasized by both the European Centre for Disease Prevention and Control and European Food Safety Authority (EFSA 2023). Campylobacteriosis was the most frequently reported zoonosis, with 137,107 cases in 2022. The treatment of infections associated with biofilms poses a particular challenge, as microbes in biofilms are up to 1000-fold more resistant to antibiotics than their planktonic counterparts (Rossi et al. 2021). Consequently, pathogenic biofilms contribute to persistent contamination and repeated infections and exacerbate the problem of antimicrobial resistance in the food industry (Paul et al. 2022). Biofilms function as multi-layered protective networks, and investigating the intricate and resilient nature of C. jejuni biofilms requires a comprehensive approach. Each cell within these communities plays a distinct role and is characterized by different properties that contribute to the overall function and resilience of the biofilm (Tram et al. 2020; Ma et al. 2022). The recurrent formation of biofilms is closely linked to the transition of bacteria to the VBNC state. This complex relationship emphasizes the importance of understanding the different roles and characteristics of individual cells within biofilms to effectively monitor and control persistent and resistant forms of C. jejuni.
In the current study, a plasmid construct containing the NanoLuc luciferase gene (pMW10_nLuc) was developed and successfully introduced into C. jejuni, resulting in a new bioluminescent C. jejuni NanoLuc strain. This method was validated by demonstrating increasing bioluminescence values with increasing cell densities. The limit of detection and limit of quantification were determined to be 9.12 (± 2.13) × 106 and 1.61 (± 0.48) × 107 CFU/mL, respectively. Although this method is effective, it is less sensitive than the Listeria innocua bioluminescence-based method described by Berlec et al. (2021). Nevertheless, the accuracy and precision of the method were within acceptable ranges: the relative error remained below 30% for most samples, and the precision (indicated by the coefficient of variation) was below 11% at higher concentrations and below 19% at lower concentrations. Crucially, the technique demonstrated reliable repeatability on consecutive days.
We used the C. jejuni NanoLuc strain together with bioluminescence assays to monitor the evolving state of biofilm after 4, 8, 24, 48, and 72 h. We compared the results of bioluminescence with the results of established biofilm quantification methods: crystal violet staining, plate counting, and resazurin fluorescence assays. We also used the bioluminescence method to monitor C. jejuni biofilm formation on both abiotic and biotic surfaces. Abiotic conditions were mimicked using polystyrene microtiter plates, whereas biotic conditions were mimicked by coating the same type of plates with different mucin concentrations. The process of initial cell adhesion and subsequent biofilm development showed similar patterns on both surfaces, with cells already adhering within the first few hours. This observation is consistent with the findings of Moe et al. (2010), who reported that the structural organization of Campylobacter biofilm becomes apparent already after 6 h.
After 4 and 8 h, the biofilm cell numbers on polystyrene surfaces determined by plate counting were similar or even lower than those determined by bioluminescence. With increasing incubation times from 24 to 72 h, cell numbers determined by bioluminescence measurements exceeded those determined by plate counting. The number of culturable cells identified using the plate counting method increased after 24 h and remained high for up to 48 h. After 72 h, however, the detectable number of culturable cells significantly decreased. These findings suggest that the critical period for C. jejuni biofilm formation is 24 h. After 48 and 72 h, the biofilm enters a mature or late phase, consistent with definitions that emphasize specific features related to the composition of extracellular polymeric substances (Ma et al. 2022) and that is characterized by cell death and dynamic processes of attachment and detachment, maintaining a relatively stable total cell number.
In our study, the differences in biofilm cell numbers between different mucin concentrations were less pronounced than expected. Notably, bioluminescence assays revealed that biofilms on mucin surfaces were more abundant than those on polystyrene, underscoring the important role of protein-glycan interactions in facilitating bacterial adhesion and invasion in the gastrointestinal tract (Alemka et al. 2012; Linden et al. 2008; Sabotič et al. 2023). After 72 h, the plate counting method revealed a decrease in culturable cells on mucin surfaces, similar to the observations on polystyrene surfaces. The data suggest that as biofilms mature (characterized by outer cells detaching and floating freely after two to 3 days), cells within the biofilm simultaneously transform into non-culturable states, including VBNC forms. However, these transformed cells remain detectable by bioluminescence assays, underscoring the effectiveness of this method in identifying different cell states during biofilm development.
In this study, crystal violet staining indicated an increase in biofilm biomass over time due to the accumulation of extracellular polymers rather than an increase in cell numbers within mature biofilms. This observation is consistent with the challenges of counting individual cells in immature biofilms, which is labor-intensive and prone to bias and becomes even more complicated in mature biofilms due to their three-dimensional structure (Wilson et al. 2017).
We also measured fluorescence using resazurin, which quantifies cells that are metabolically active. After 48 and 72 h, fluorescence rates slightly decreased compared to that after 24 h. This subtle decrease implies that despite the decrease in cell number in the mature phase after the initial increase in cell number, the metabolic activity of the cells was not significantly changed. This trend is consistent with the observations made with different biofilm quantification methods over time, which vary depending on the initial inoculum concentrations (Supplementary Figure S3).
The negative trends observed with plate counting and resazurin fluorescence indicate a decrease in the culturability and metabolic activity of biofilm cells. This implies that methods relying on metabolic activity (e.g., resazurin assays) can provide inaccurate results for adherent cells (Klančnik et al. 2021) and thus potentially also biofilm cells. By contrast, the positive trends observed with NanoLuc and crystal violet staining indicate an increase in total cell count and biomass. Crystal violet and resazurin staining methods have relatively lower sensitivity and higher standard deviations compared to plate counting and bioluminescence methods, emphasizing the importance of using multiple techniques for a comprehensive quantitative analysis of biofilm formation. The differences in sensitivity and precision between methods indicate that no single method can cover all aspects of biofilm characteristics; instead, the combination of different techniques provides unique insights into biofilm behavior.
To simulate conditions similar to real food, chicken meat juice was used as the cultivation medium, replacing conventional MH broth. Bioluminescence and plate counting revealed significantly higher numbers of attached and biofilm-forming cells in chicken juice than those in MH broth. Chicken juice contains nutrient-rich particles that can form a protective layer on surfaces, facilitating the initial adhesion and subsequent biofilm formation of bacteria such as Campylobacter (Li et al. 2017). This synergy between the nutritive properties of chicken juice and its role in improving surface conditions highlights the complex interplay of factors that contribute to biofilm development on different materials (Brown et al. 2014; Li et al. 2017). In multispecies environments, the presence of other bacterial species can significantly influence the growth and biofilm formation of pathogenic bacteria, and this requires specific detection methods (Ica et al. 2012).
Our quantification of C. jejuni biofilm cells in a mixed culture with pathogenic S. enterica with the bioluminescence method revealed a higher number of cells compared to the plate counting method. This is an indication of the effectiveness of bioluminescence measurement in detecting biofilm formation in a competitive multispecies environment. This highlights the complex dynamics of bacterial interactions and their impact on biofilm formation and pathogen persistence.
The value of the NanoLuc bioluminescence method lies in its sensitivity and specificity as well as technical simplicity and speed. This combination enables the specific detection of C. jejuni cells expressing the NanoLuc enzyme, enabling holistic monitoring of biofilm formation. This capability is particularly critical for comprehensive studies of biofilm dynamics, as it provides insight into the behavior of C. jejuni under different conditions, supporting effective strategies for dealing with biofilms in both clinical and food safety contexts.
Table 3 shows a comparative analysis of the NanoLuc method under different experimental conditions. This includes an assessment of biofilm state (early/mature), mixed biofilms, biofilm thickness, surface type, and dynamic biofilms for all four methods: plate counting (CFU/mL), NanoLuc bioluminescence assay, crystal violet assay, and resazurin. Bioluminescence is a very sensitive and selective method with low background signal, making it suitable for use in complex biofilm environments. It can detect VBNC forms and damaged but not lysed cells and specifically identify organisms expressing the NanoLuc protein, which is advantageous for studying various microbial or multi-species biofilms altogether. When comparing methods for monitoring biofilms, NanoLuc bioluminescence stands out as an effective technique due to its ability to detect different phenotypic subpopulations within biofilms. This capability is particularly important for the study of Campylobacter as it allows the identification of combined culturable cells, VBNC forms and damaged but not lysed cells together. However, it should be emphasized that this method alone does not make it possible to differentiate between these various cell states that may be present in biofilms. In dead cells, the signal initially remains stable but is transient; the luminescence fades as soon as the cells are completely degraded and NanoLuc is inactivated.
Table 3.
A comparative analysis of the NanoLuc method under different experimental conditions
| Plate counting (CFU/mL) (Klančnik et al. 2017a) | NanoLuc bioluminescence assay (this manuscript) | Crystal violet assay (Kurinčič et al. 2016; Klančnik et al. 2017a) | Resazurin (Kovač et al. 2015) | |
|---|---|---|---|---|
| Biofilm state (early/mature biofilm) | Less suitable for mature biofilm. Increase in damaged, VBNC and dead cells that cannot be cultured results in underestimation of cell number | Enables the detection of culturable, VBNC and damaged cells, and is therefore suitable for various biofilm conditions | Increase in biofilm biomass over time due to the accumulation of extracellular polymers and not due to an increase in the number of cells in mature biofilms; inaccurate for mature biofilms | Detects metabolically active cells. Suitable for various biofilm conditions |
| Mixed biofilms | Selection method, such as antibiotic resistance, is required for the identification of individual organisms | Suitable for mixed biofilms. Only organisms with the expressed NanoLuc protein can be detected | The distinction between organisms is not possible. Only the total biomass can be determined | The distinction between organisms is not possible. Only the general metabolic activity can be determined |
| Biofilm thickness | Problem with detachment and degradation of the biofilm, cells could be present in clusters | Limited substrate penetration into the thick biofilm and signal attenuation due to the multilayer biofilm | Limited dye penetration into the thick biofilm | Limited substrate penetration into the thick biofilm and signal attenuation due to the multilayer biofilm |
| Type of surface used for biofilm growth | Difficult removal of biofilms from rough surfaces | Risk of light diffusion on certain surfaces | The rinsing protocol must be adapted to the surface used | Risk of light diffusion on certain surfaces |
| Dynamic biofilm | Detachment of cells in the flow-through system results in the destruction of the dynamic biofilm | Retention of the substrate prevents continuous monitoring. Intervals between measurements should be considered | Rinsing results in the destruction of the dynamic biofilm | Retention of the substrate prevents continuous monitoring. Intervals between measurements should be considered |
The transition of Campylobacter to the VBNC state is triggered by several stress factors that occur during food production and processing. These conditions often lead to the accumulation of organic and inorganic matter, which favors the development of resistant biofilms and enables bacteria to survive in hostile environments. This represents a significant challenge for both clinical management and public health efforts, as emphasized by Carrascosa et al. (2021). Recognizing the importance of VBNC states in the analysis of Campylobacter is therefore critical, as these states have a profound impact on detection methods and research results. As such, advanced detection methods such as NanoLuc bioluminescence should be incorporated into studies on the complex dynamics of bacterial biofilms, particularly in the context of mitigating public health risks associated with foodborne pathogens.
In contrast to NanoLuc, plate counting is an indirect method that focuses exclusively on live culturable cells. Crystal violet and resazurin also offer direct detection, with crystal violet detecting all components of the biofilm, and resazurin targeting living metabolically active cells. NanoLuc combined with measurements of culturable cells by plate counts and metabolic activity by resazurin provides absolute cell quantification, in contrast to the relative quantification of crystal violet.
One of the downsides od this methosd is C. jejuni requiring genetic modification to express the NanoLuc enzyme. In complex samples, other luminescent signals or quenching effects could interfere with the detection of the specific bioluminescent signal of NanoLuc-expressing C. jejuni, requiring additional controls. In terms of operating costs and time, a plate reader is required for NanoLuc as well as crystal violet and resazurin tests. Conversely, plate counting is more time-consuming but does not require specialized equipment.
Our findings have important implications for food safety and public health, particularly for controlling the spread of Campylobacter in poultry. By contaminating chicken meat with luminescently labelled Campylobacter, we could track the transmission of the pathogen through the entire food processing chain—from poultry to equipment and packaging to consumers’ kitchens. This study highlights the potential for biofilm formation at various stages of food processing, with the bacteria persisting in a viable but non-culturable (VBNC) state. Using molecular methods, we could monitor the presence and persistence of these bacteria, providing important insights into contamination sites. These findings can help to develop better hygiene practices and intervention strategies to reduce foodborne infections.
The developed technology has proven useful in various applications, including cell biology, the study of microbial interactions, cell signaling, protein–protein interactions, gene regulation, and protein stability (England et. al, 2016; Shang et. al, 2023; Chen et. al, 2015; Russo et. al, 2022). These applications can be specifically tailored to the C. jejuni strain developed in this study.
In summary, the NanoLuc bioluminescence assay represents an advance in monitoring and understanding C. jejuni biofilms by enabling non-invasive assessment of bacterial communities without the need for physical cell manipulation. Its specificity, sensitivity, and technical simplicity emphasize its potential as a tool for specific C. jejuni detection and holistic biofilm monitoring, which could improve our understanding of biofilm development, structural integrity, and resistance mechanisms. By allowing in situ application, this method preserves the natural conditions of biofilms, which is essential for studying their natural development and resistance to cleaning and disinfection. In addition, the advantages of the assay, including direct detection, absolute cell quantification, minimal time required, and high sensitivity, represent a significant alternative or complement to conventional biofilm quantification techniques. Its ability to quantify all cell types within biofilms, including VBNC cells, underscores its importance for public health, particularly regarding C. jejuni infectivity and survival strategies. However, the limitations of the approach, such as detection thresholds, the need for genetic modification, and the relative cost, point to the importance of an integrated methodology. Future studies should aim to validate the NanoLuc assay under different real-life conditions and explore its applicability in vivo to expand its utility in infection studies and clinical diagnostics. The NanoLuc bioluminescence assay shows promise to improve our understanding of biofilms, with significant implications for public health, food safety, and beyond.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We thank Manca Volk for technical support and Dr. Eva Lasic for editing and reviewing the manuscript.
Author contribution
TČ conducted experiments, analyzed data, and wrote the original draft; PŠ assisted with molecular cloning; OS and QZ assisted with the methodology and reviewed and improved the manuscript; AB conceived and designed the study, assisted with the methodology, and reviewed the manuscript; AK conceived and designed the study and was responsible for the overall coordination of this study and the manuscript. All authors have read and approved the final manuscript.
Funding
This work was supported by the Slovenian Research and Innovation Agency (grant numbers J4-3088, J4-4548, J4-4550, J7-4420, P4-0116, and BI-US/22–24-073).
Data availability
All data supporting the results of this study are included in the publication and the supplementary document. The raw data are available on request from the corresponding author.
Declarations
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Alemka A, Corcionivoschi N, Bourke B (2012) Defense and adaptation: the complex inter-relationship between Campylobacter jejuni and mucus. Front Cell Infect Microbiol 2:15. 10.3389/fcimb.2012.00015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alves A, Brito E, Ferreira F, Celestino J (2020) Methods for studying microbial biofilm. Int J Dev Res 10(12):43100–43104. 10.37118/ijdr.20601.12.2020 [Google Scholar]
- Azeredo J, Azevedo NF, Briandet R, Cerca N, Coenye T, Costa AR, Desvaux M, Di Bonaventura G, Hébraud M, Jaglic Z, Kačániová M, Knøchel S, Lourenço A, Mergulhão F, Meyer RL, Nychas G, Simões M, Tresse O, Sternberg C (2017) Critical review on biofilm methods. Crit Rev Microbiol. 10.1080/1040841X.2016.1208146 [DOI] [PubMed]
- Berlec A, Janež N, Sterniša M, Klančnik A, Sabotič J (2021) Expression of NanoLuc luciferase in Listeria innocua for development of biofilm assay. Front Microbiol 12:636421. 10.3389/fmicb.2021.636421 [DOI] [PMC free article] [PubMed]
- Berlec A, Janež N, Sterniša M, Klančnik A, Sabotič J (2022) Listeria innocua biofilm assay using NanoLuc luciferase. Bio Protoc 12(3):e4308. 10.21769/BioProtoc.4308 [DOI] [PMC free article] [PubMed]
- Brown HL, Reuter M, Salt LJ, Cross KL, Betts RP, van Vliet AHM (2014) Chicken juice enhances surface attachment and biofilm formation of Campylobacter jejuni. Appl Environ Microbiol 80(22):7053–7060. 10.1128/AEM.02778-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrascosa C, Raheem D, Ramos F, Saraiva A, Raposo A (2021) Microbial biofilms in the food industry—a comprehensive review. Int J Environ Res Public Health 18(4):2014. 10.3390/ijerph18042014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Bagdasarian M, Walker ED (2015) Elizabethkingia anophelis: molecular manipulation and interactions with mosquito hosts. Appl Environ Microbiol 81:2233–2243. 10.1128/AEM.03733-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis L, Young K, DiRita V (2008) Genetic manipulation of Campylobacter jejuni. Curr Protoc Microbiol 10: 8A.2.1–8A.2.17. 10.1002/9780471729259.mc08a02s10 [DOI] [PMC free article] [PubMed]
- Duarte A, Alves A, Ferreira S, Silva F, Domingues F (2015) Resveratrol inclusion complexes: antibacterial and anti-biofilm activity against Campylobacter spp. and Arcobacter butzleri. Food Res Int 77(2):244–250. 10.1016/j.foodres.2015.05.047
- EFSA, ECDC (2023) The European Union One Health 2022 Zoonoses Report. EFSA J 21(12):e8442. 10.2903/j.efsa.2023.8442 [DOI] [PMC free article] [PubMed]
- Elgamoudi BA, Ketley JM (2018) Lighting up my life: a LOV-based fluorescent reporter for Campylobacter jejuni. Res Microbiol. 10.1016/j.resmic.2017.10.003 [DOI] [PubMed] [Google Scholar]
- England CG, Ehlerding EB, Cai W (2016) NanoLuc: a small luciferase is brightening up the field of bioluminescence. Bioconjug Chem 27(5):1175–1187. 10.1021/acs.bioconjchem.6b00112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin MJ, Chang C, Akiyama T, Bothner B (2015) New technologies for studying biofilms. Microbiol Spectr 3(4). 10.1128/microbiolspec.MB-0016-2014 [DOI] [PMC free article] [PubMed]
- Giaouris E, Heir E, Desvaux M, Hébraud M, Møretrø T, Langsrud S, Doulgeraki A, Nychas GJ, Kačániová M, Czaczyk K, Ölmez H, Simões M (2015) Intra- and inter-species interactions within biofilms of important foodborne bacterial pathogens. Front Microbiol 6:841. 10.3389/fmicb.2015.00841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good L, Miller WG, Niedermeyer J, Osborne J, Siletzky RM, Carver D, Kathariou S (2019) Strain-specific differences in survival of Campylobacter spp. in naturally contaminated turkey feces and water. Appl Environ Microbiol 85(18):e01579-19. 10.1128/AEM.01579-19 [DOI] [PMC free article] [PubMed]
- Hall MP, Unch J, Binkowski BF, Valley MP, Butler BL, Wood MG, Otto P, Zimmerman K, Vidugiris G, Machleidt T, Robers MB, Benink HA, Eggers CT, Slater MR, Michael R, Meisenheimer PL, Klaubert DH, Fan F, Encell LP, Wood KH (2012) Engineered luciferase reporter from a deep sea shrimp utilizing a novel imidazopyrazinone substrate. ACS Chem Biol 7(11):1848–1857. 10.1021/cb3002478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermans D, Pasmans F, Messens W, Martel A, Van Immerseel F, Rasschaert G, Heyndrickx M, Van Deun K, Haesebrouck F (2012) Poultry as a host for the zoonotic pathogen Campylobacter jejuni. Vector Borne Zoonotic Dis 12:89–98. 10.1128/aem.01579-19 [DOI] [PubMed] [Google Scholar]
- Ica T, Caner V, Istanbullu O, Nguyen HD, Ahmed B, Call DR, Beyenal H (2012) Characterization of mono- and mixed-culture Campylobacter jejuni biofilms. Appl Environ Microbiol 78(4):1033–1038. 10.1128/AEM.07364-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ijaz UZ, Sivaloganathan L, McKenna A, Richmond A, Kelly C, Linton M, Stratakos AC, Lavery U, Elmi A, Wren B, Dorrell N, Gundogdu O (2018) Comprehensive longitudinal microbiome analysis of the chicken cecum reveals a shift from competitive to environmental drivers and a window of opportunity for Campylobacter. Front Microbiol 9:2452. 10.3389/fmicb.2018.02452 [DOI] [PMC free article] [PubMed]
- Jackson DN, Davis B, Tirado SM, Duggal M, van Frankenhuyzen JK, Deaville D, Wijesinghe MAK, Tessaro M, Trevors JT (2009) Survival mechanisms and culturability of Campylobacter jejuni under stress conditions. Antonie Van Leeuwenhoek 96(4):377–394. 10.1007/s10482-009-9378-8 [DOI] [PubMed] [Google Scholar]
- Jug B, Šikić Pogačar M, Sterniša M, Tumpej T, Karničar K, Turk D, Langerholc T, Sabotič J, Klančnik A (2024) Modulation of Campylobacter jejuni adhesion to biotic model surfaces by fungal lectins and protease inhibitors. Front Cell Infect Microbiol 14:1391758. 10.3389/fcimb.2024.1391758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klančnik A, Guzej B, Jamnik P, Vucković D, Abram M, Smole Možina S (2009) Stress response and pathogenic potential of Campylobacter jejuni cells exposed to starvation. Res Microbiol 160:345–352. 10.1016/j.resmic.2009.05.002 [DOI] [PubMed] [Google Scholar]
- Klančnik A, Vučković D, Jamnik P, Abram M, Smole Možina S (2014) Stress response and virulence of heat-stressed Campylobacter jejuni. Microbes Environ 29:338–345. 10.1264/jsme2.ME14020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovač J, Šimunović K, Wu Z, Klančnik A, Bucar F, Zhang Q, Smole Možina S (2015) Antibiotic resistance modulation and modes of action of (-)-α-pinene in Campylobacter jejuni. PLoS ONE 10(4):e0122871. 10.1371/journal.pone.0122871 [DOI] [PMC free article] [PubMed]
- Kurinčič M, Jeršek B, Klančnik A, Smole Možina S, Fink R, Dražić G, Raspor P, Bohinc K (2016) Effects of natural antimicrobials on bacterial cell hydrophobicity, adhesion, and zeta potential. Arch Ind Hyg Toxicol 67(1):39–45. 10.1515/aiht-2016-67-2720 [DOI] [PubMed]
- Klančnik A, Šikić Pogačar M, Trošt K, Tušek Žnidarič M, Mozetič Vodopivec B, Smole Možina S (2017a) Anti-Campylobacter activity of resveratrol and an extract from waste Pinot noir grape skins and seeds, and resistance of Camp. jejuni planktonic and biofilm cells, mediated via the CmeABC efflux pump. J Appl Microbiol 122(1):65–77. 10.1111/jam.13315 [DOI] [PubMed]
- Klančnik A, Zorko Š, Toplak N, Kovač M, Bucar F, Jeršek B, Smole Možina S (2017b) Antiadhesion activity of juniper (Juniperus communis L.) preparations against Campylobacter jejuni evaluated with PCR-based methods. Phytother Res 32(3):542–550. 10.1002/ptr.6005 [DOI] [PubMed]
- Klančnik A, Šimunović K, Sterniša M, Ramić D, Smole Možina S, Bucar F (2021) Anti-adhesion activity of phytochemicals to prevent Campylobacter jejuni biofilm formation on abiotic surfaces. Phytochem Rev 20:55–84. 10.1007/s11101-020-09669-6 [Google Scholar]
- Li J, Feng J, Ma L, de la Fuente NC, Gölz G, Lu X (2017) Effects of meat juice on biofilm formation of Campylobacter and Salmonella. Int J Food Microbiol 253:20–28. 10.1016/j.ijfoodmicro.2017.04.013 [DOI] [PubMed] [Google Scholar]
- Linden SK, Sutton P, Karlsson NG, Korolik V, McGuckin MA (2008) Mucins in the mucosal barrier to infection. Mucosal Immunol 1(3):183–197. 10.1038/mi.2008.5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L, Feng J, Zhang J, Lu X (2022) Campylobacter biofilms. Microbiol Res 264:127149. 10.1016/j.micres.2022.127149 [DOI] [PubMed] [Google Scholar]
- Miller WG, Bates AH, Horn ST, Brandl MT, Wachtel MR, Mandrell RE (2000) Detection on surfaces and in Caco-2 cells of Campylobacter jejuni cells transformed with new GFP, YFP, and CFP marker plasmids. Appl Environ Microbiol 66:5426–5436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moe KK, Mimura J, Ohnishi T, Wake T, Yamazaki W, Nakai M, Misawa N (2010) The mode of biofilm formation on smooth surfaces by Campylobacter jejuni. J Vet Med Sci 72(4):411–416. 10.1292/jvms.09-0339 [DOI] [PubMed] [Google Scholar]
- Oh E, Andrews KJ, Jeon B (2018) Enhanced biofilm formation by ferrous and ferric iron through oxidative stress in Campylobacter jejuni. Front Microbiol 9:1204. 10.3389/fmicb.2018.01204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul M, Carrara E, Retamar P, Tängdén T, Bitterman R, Bonomo RA, de Waele J, Daikos GL, Akova M, Harbarth S, Pulcini C, Garnacho-Montero J, Seme K, Tumbarello M, Lindemann PC, Gandra S, Yu Y, Bassetti M, Mouton JW, Tacconelli E, Rodríguez-Baño J (2022) European Society of Clinical Microbiology and Infectious Diseases (ESCMID) guidelines for the treatment of infections caused by multidrug-resistant Gram-negative bacilli (endorsed by European Society of Intensive Care Medicine). Clin Microbiol Infect 28(4):521–547. 10.1016/j.cmi.2021.11.025 [DOI] [PubMed] [Google Scholar]
- Piskernik S, Klančnik A, Riedel TC, Brøndsted L, Smole Možina S (2011) Reduction of Campylobacter jejuni by natural antimicrobials in chicken-meat-related conditions. Food Control 22(5):718–724. 10.1016/j.foodcont.2010.11.002 [Google Scholar]
- Ramamurthy T, Ghosh A, Pazhani GP, Shinoda S (2014) Current perspectives on viable but non-culturable (VBNC) pathogenic bacteria. Front Public Health 2:103. 10.3389/fpubh.2014.00103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramić D, Bucar F, Kunej U, Dogša I, Klančnik A, Smole Možina S (2021) Antibiofilm potential of Lavandula preparations against Campylobacter jejuni. Appl Environ Microbiol 87(19):e01099-21. 10.1128/AEM.01099-21 [DOI] [PMC free article] [PubMed]
- Rossi DA, Dumont CF, Santos ACS, Vaz MEL, Prado RR, Monteiro GP, Melo CBS, Stamoulis VJ, Santos JP, Melo RT (2021) Antibiotic resistance in the alternative lifestyles of Campylobacter jejuni. Front Cell Infect Microbiol 11. 10.3389/fcimb.2021.535757 [DOI] [PMC free article] [PubMed]
- Russo DA, Zedler JAZ, Conradi FD, Schuergers N, Jensen PE, Mullineaux CW, Wilde A, Pohnert G (2022) Development of a highly sensitive luciferase-based reporter system to study two-step protein secretion in Cyanobacteria. J Bacteriol 204. 10.1128/jb.00504-21 [DOI] [PMC free article] [PubMed]
- Sabotič J, Janež N, Volk M, Klančnik A (2023) Molecular structures mediating adhesion of Campylobacter jejuni to abiotic and biotic surfaces. Vet Microbiol. 10.1016/j.vetmic.2023.109918 [DOI] [PubMed] [Google Scholar]
- Shang W, Hu Z, Li M, Wang Y, Rao Y, Tan L et al (2023) Optimizing a high-sensitivity NanoLuc-based bioluminescence system for in vivo evaluation of antimicrobial treatment. mLife 2:462–478. 10.1002/mlf2.12091 [DOI] [PMC free article] [PubMed]
- Solomon EB, Hoover DG (1999) Campylobacter jejuni: a bacterial paradox. J Food Saf 19(2):121–136. 10.1111/j.1745-4565.1999.tb00239.x
- Syed AJ, Anderson JC (2021) Applications of bioluminescence in biotechnology and beyond. Chem Soc Rev 50:5668–5705. 10.1039/D0CS01492C [DOI] [PubMed] [Google Scholar]
- Teh AHT, Lee SM, Dykes GA (2014) Does Campylobacter jejuni form biofilms in food-related environments? Appl Environ Microbiol 80(17):5154–5160. 10.1128/AEM.01493-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trošt K, Klančnik A, Mozetič Vodopivec B, Sternad Lemut M, Jug Novšak K, Raspor P, Smole Možina S (2016) Polyphenol, antioxidant and antimicrobial potential of six different white and red wine grape processing leftovers. J Sci Food Agric 96(14):4809–4820. 10.1002/jsfa.7981 [DOI] [PubMed] [Google Scholar]
- Tram G, Day CJ, Korolik V (2020) Bridging the gap: a role for Campylobacter jejuni biofilms. Microorganisms 8(3):452. 10.3390/microorganisms8030452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagle BR, Upadhyay A, Upadhyaya I, Shrestha S, Arsi K, Liyanage R, Venkitanarayanan K, Donoghue DJ, Donoghue AM (2019) Trans-cinnamaldehyde, eugenol and carvacrol reduce Campylobacter jejuni biofilms and modulate expression of select genes and proteins. Front Microbiol 10:1837. 10.3389/fmicb.2019.01837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkelströter LK, Teixeira FB, Silva EP, Alves VF, De Martinis CP (2014) Unraveling microbial biofilms of importance for food microbiology. Microb Ecol 68(1):35–46. 10.1007/s00248-013-0347-4 [DOI] [PubMed] [Google Scholar]
- Wilson C, Lukowicz R, Merchant S, Valquier-Flynn H, Caballero J, Sandoval J, Okuom M, Huber C, Durham Brooks T, Wilson E, Clement B, Wentworth CD, Holmes AE (2017) Quantitative and qualitative assessment methods for biofilm growth: a mini-review. Res Rev J Eng Technol 6(4) [PMC free article] [PubMed]
- Wösten MM, Boeve M, Koot MG, Van Nuenen AC, Van der Zeijst BA (1998) Identification of Campylobacter jejuni Promoter Sequences. J Bacteriol Res 180(3):594–599. 10.1128/JB.180.3.594-599.1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data supporting the results of this study are included in the publication and the supplementary document. The raw data are available on request from the corresponding author.