Abstract
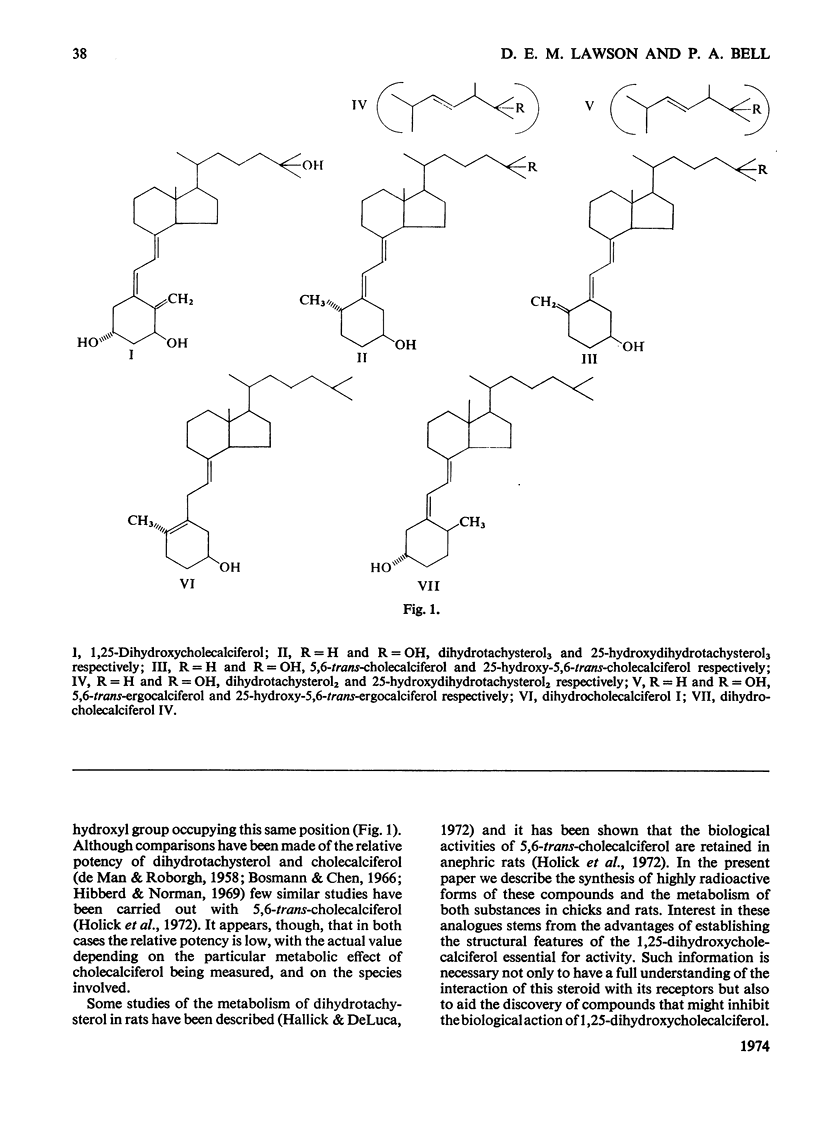
Dihydrotachysterol and 5,6-trans-cholecalciferol are biologically active analogues of cholecalciferol (vitamin D) with a similarity in steric structure to 1,25-dihydroxycholecalciferol, the active form of the vitamin. The question arises as to the nature of the active form of these analogues. High specific radioactivity 14C- and 3H-labelled forms of dihydrotachysterol and 5,6-trans-cholecalciferol and its 25-hydroxy derivative were synthesized and their metabolism was studied in chicks and rats. All these steroids were very rapidly metabolized compared with cholecalciferol; 20% of the dihydrotachysterol dose was excreted in bile in the first 24h, about 50% as a carboxylic acid derivative. Although polar metabolites were detected in tissues, no 1-hydroxy form was observed. Larger proportions of the parent steroid and its 25-hydroxy metabolite were detected in tissues compared with cholecalciferol, but no single metabolite was detected at the intracellular site of action of cholecalciferol. It is suggested that analogues of cholecalciferol will be biologically active if they possess a hydroxyl group in the same steric position as that at C-1 of cholecalciferol, with the greatest activity shown by those that also have a C-25 hydroxyl group. The implication of these findings for the chemical features necessary for binding to receptor proteins are briefly discussed.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Bell P. A., Kodicek E. The stereospecificity of tritium distribution in [1-3H]- and [1,2-3H2]-cholesterol and -cholecalciferol. Biochem J. 1970 Feb;116(4):755–757. doi: 10.1042/bj1160755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bontekoe J. S., Wignall A., Rappoldt M. P., Roborgh J. R. Hydroxylated vitamin D analogues. Int Z Vitaminforsch. 1970;40(5):589–591. [PubMed] [Google Scholar]
- Bosmann H. B., Chen P. S., Jr Comparative effects of dihydrotachysterol-2 and dihydrotachysterol-3 in chicks. J Nutr. 1966 Oct;90(2):141–147. doi: 10.1093/jn/90.2.141. [DOI] [PubMed] [Google Scholar]
- DeLuca H. F., Suda T., Schnoes H. K., Tanaka Y., Holick M. F. 25,26-dihydroxycholecalciferol, a metabolite of vitamin D3 with intestinal calcium transport activity. Biochemistry. 1970 Nov 24;9(24):4776–4780. doi: 10.1021/bi00826a022. [DOI] [PubMed] [Google Scholar]
- Fraser D. R., Kodicek E. Unique biosynthesis by kidney of a biological active vitamin D metabolite. Nature. 1970 Nov 21;228(5273):764–766. doi: 10.1038/228764a0. [DOI] [PubMed] [Google Scholar]
- Gray R., Boyle I., DeLuca H. F. Vitamin D metabolism: the role of kidney tissue. Science. 1971 Jun 18;172(3989):1232–1234. doi: 10.1126/science.172.3989.1232. [DOI] [PubMed] [Google Scholar]
- Hallick R. B., DeLuca H. F. Metabolites of dihydrotachysterol in target tissues. J Biol Chem. 1972 Jan 10;247(1):91–97. [PubMed] [Google Scholar]
- Hibberd K. A., Norman A. W. Comparative biological effects of vitamins D 2 and D 3 and dihydrotachysterol 2 and dihydrotachysterol 3 in the chick. Biochem Pharmacol. 1969 Oct;18(10):2347–2355. doi: 10.1016/0006-2952(69)90349-9. [DOI] [PubMed] [Google Scholar]
- Holick M. F., Garabedian M., DeLuca H. F. 5,6-Trans isomers of cholecalciferol and 25-hydroxycholecalciferol. Substitutes for 1,25-dihydroxycholecalciferol in anephric animals. Biochemistry. 1972 Jul 4;11(14):2715–2719. doi: 10.1021/bi00764a026. [DOI] [PubMed] [Google Scholar]
- Holick M. F., Schnoes H. K., DeLuca H. F., Suda T., Cousins R. J. Isolation and identification of 1,25-dihydroxycholecalciferol. A metabolite of vitamin D active in intestine. Biochemistry. 1971 Jul 6;10(14):2799–2804. doi: 10.1021/bi00790a023. [DOI] [PubMed] [Google Scholar]
- Lawson D. E., Fraser D. R., Kodicek E., Morris H. R., Williams D. H. Identification of 1,25-dihydroxycholecalciferol, a new kidney hormone controlling calcium metabolism. Nature. 1971 Mar 26;230(5291):228–230. doi: 10.1038/230228a0. [DOI] [PubMed] [Google Scholar]
- Lawson D. E., Pelc B., Bell P. A., Wilson P. W., Kodicek E. Synthesis of (1,2- 3 H 2 )cholecalciferol and metabolism of (4- 14 C,1,2- 3 H 2 )- and (4- 14 C,1- 3 H)-cholecalciferol in rachitic rats and chicks. Biochem J. 1971 Feb;121(4):673–682. doi: 10.1042/bj1210673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson D. E., Wilson P. W., Kodicek E. Metabolism of vitamin D. A new cholecalciferol metabolite, involving loss of hydrogen at C-1, in chick intestinal nuclei. Biochem J. 1969 Nov;115(2):269–277. doi: 10.1042/bj1150269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NUMEROF P., SASSAMAN H. L., RODGERS A., SCHAEFER A. E. The use of radioactive phosphorus in the assay of vitamin D. J Nutr. 1955 Jan;55(1):13–21. doi: 10.1093/jn/55.1.13. [DOI] [PubMed] [Google Scholar]
- Norman A. W., Myrtle J. F., Midgett R. J., Nowicki H. G., Williams V., Popják G. 1,25-dihydroxycholecalciferol: identification of the proposed active form of vitamin D3 in the intestine. Science. 1971 Jul 2;173(3991):51–54. doi: 10.1126/science.173.3991.51. [DOI] [PubMed] [Google Scholar]
- Norman A. W. The mode of action of vitamin D. Biol Rev Camb Philos Soc. 1968 Feb;43(1):97–137. doi: 10.1111/j.1469-185x.1968.tb01111.x. [DOI] [PubMed] [Google Scholar]
- Pelc B., Kodicek E. Synthesis of (4- 14 C) ergosterol and (4- 14 C) ergocalciferol. J Chem Soc Perkin 1. 1972;23:2980–2981. doi: 10.1039/p19720002980. [DOI] [PubMed] [Google Scholar]


