Abstract
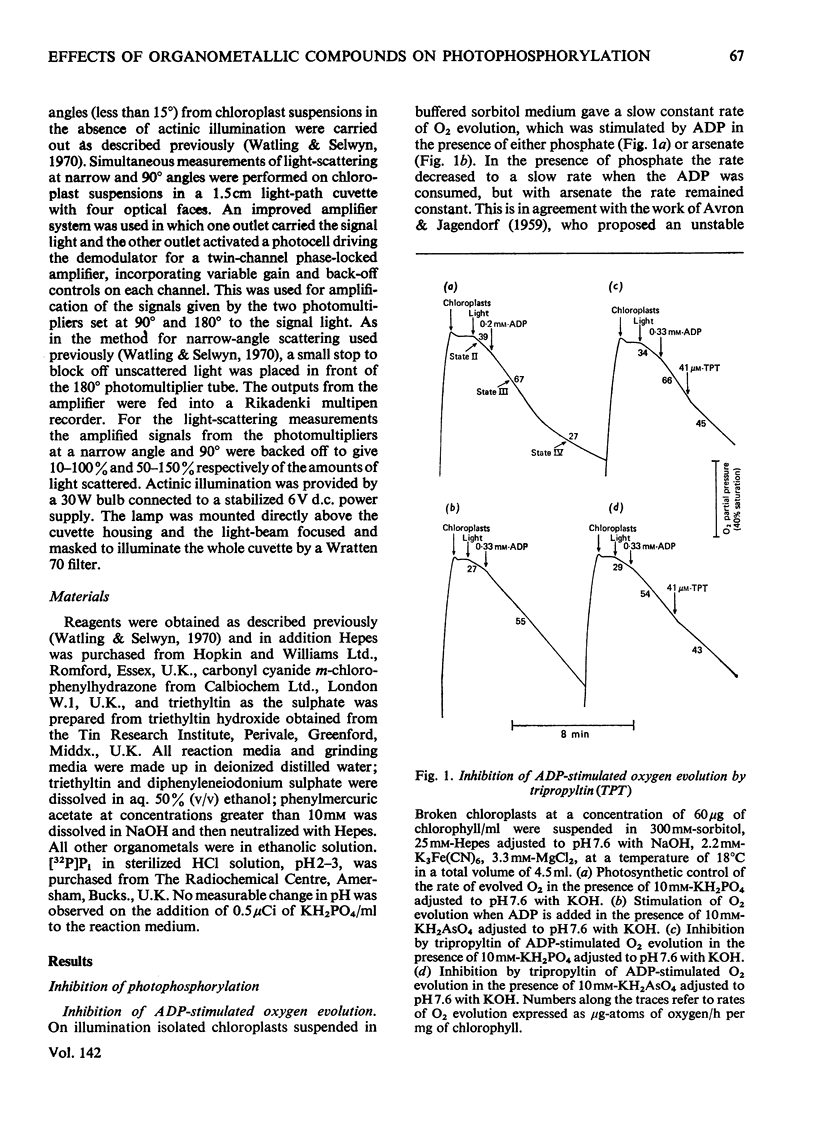
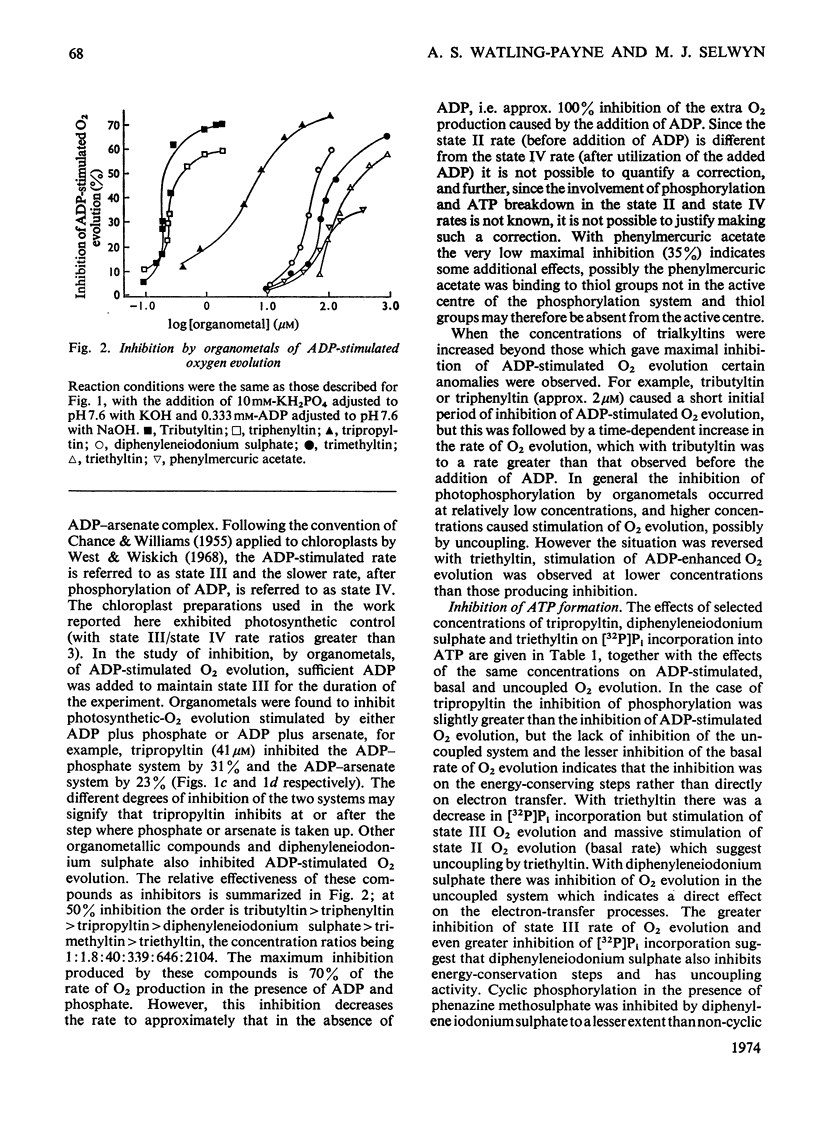
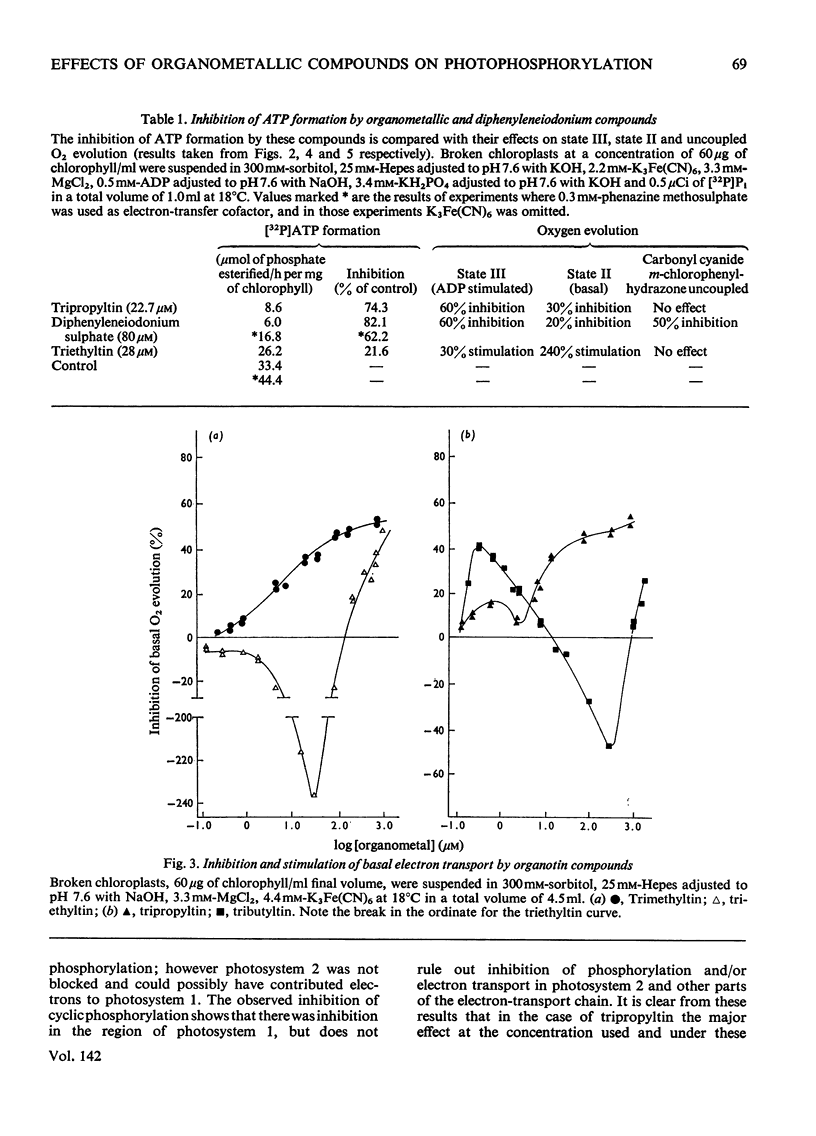
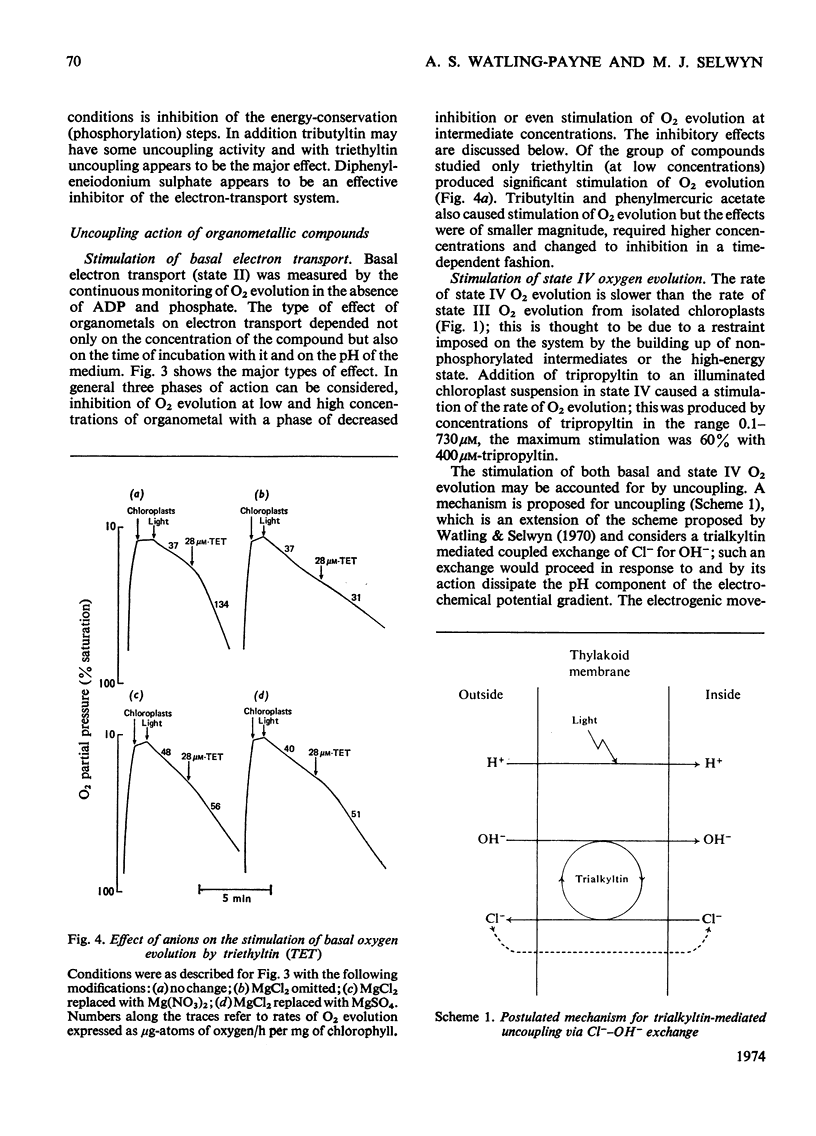
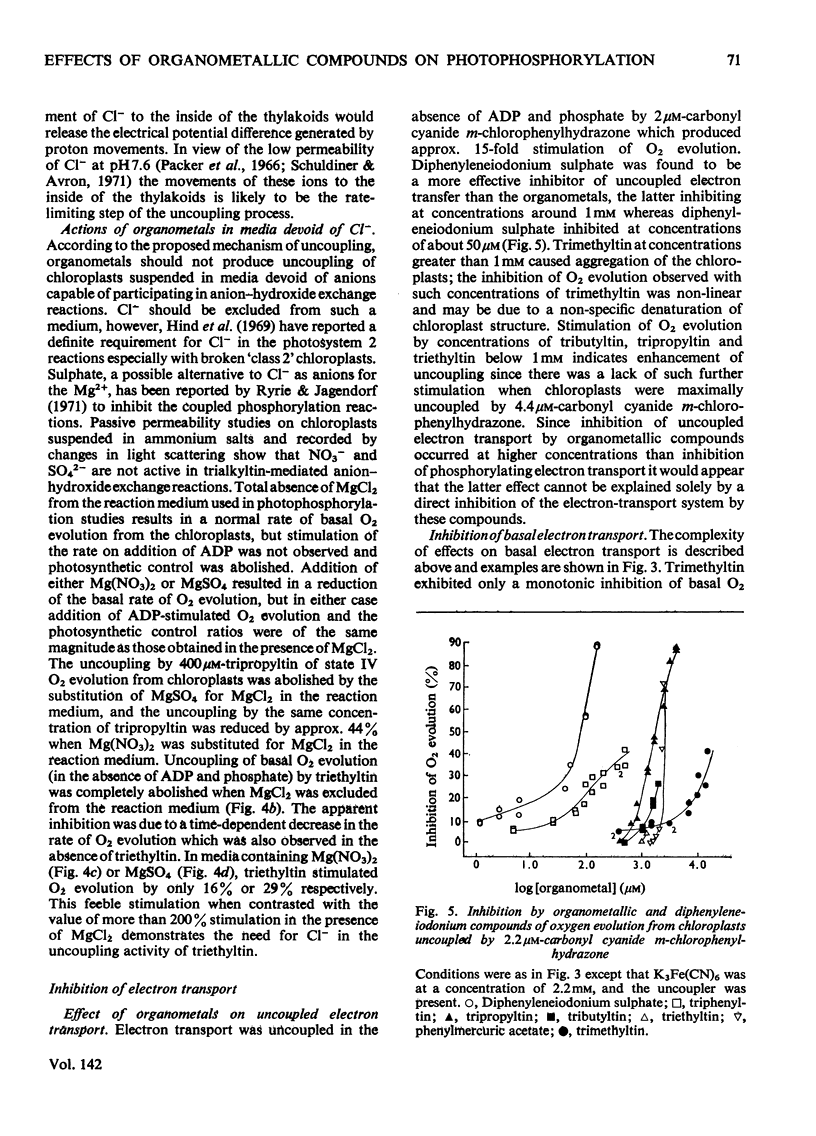
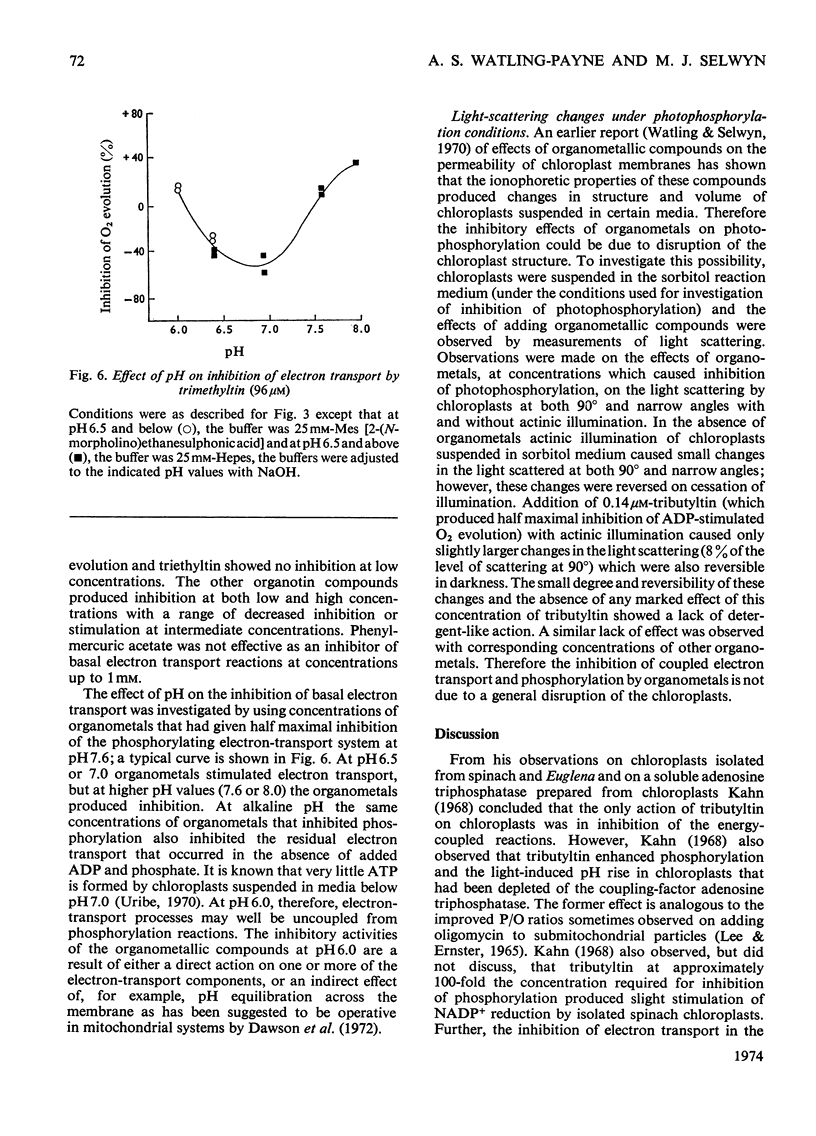
1. Trialkyltin, triphenyltin and diphenyleneiodonium compounds inhibited ADP-stimulated O2 evolution by isolated pea chloroplasts in the presence of phosphate or arsenate. Tributyltin and triphenyltin were the most effective inhibitors, which suggests a highly hydrophobic site of action. Phenylmercuric acetate was a poor inhibitor of photophosphorylation, which suggests that thiol groups are not involved. 2. Triethyltin was a potent uncoupler of photophosphorylation by isolated chloroplasts in media containing Cl−, but had little uncoupling activity when Cl− was replaced by NO3− or SO42−, which are inactive in the anion–hydroxide exchange. It is suggested that uncoupling by triethyltin is a result of the Cl−–OH− exchange together with a natural uniport of Cl−. Tributyltin, triphenyltin and phenylmercuric acetate had low uncoupling activity, probably because in these compounds the uncoupling activity is partially masked by inhibitory effects. 3. At high concentrations the organotin compounds caused inhibition of electron transport uncoupled by carbonyl cyanide m-chlorophenylhydrazone or NH4Cl. At these high concentrations the organotin compounds may be producing a detergent-like disorganization of the membrane structure. In contrast, diphenyleneiodonium sulphate inhibited uncoupled electron transport at low concentrations; however, this inhibition is less than the inhibition of photophosphorylation, which suggests that the compound also inhibits the phosphorylation reactions as well as electron transport. 4. The effects of these compounds on basal electron transport were complex and depended on the pH of the reaction media. However, they can be explained on the basis of three actions: inhibition of the phosphorylation reactions, uncoupling and direct inhibition of electron transport. 5. The inhibition of cyclic photophosphorylation in the presence of phenazine methosulphate by diphenyleneiodonium sulphate shows that it inhibits in the region of photosystem 1.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALDRIDGE W. N. The biochemistry of organotin compounds: trialkyltins and oxidative phosphorylation. Biochem J. 1958 Jul;69(3):367–376. doi: 10.1042/bj0690367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AVRON M., JAGENDORF A. T. Evidence concerning the mechanism of adenosine triphosphate formation by spinach chloroplasts. J Biol Chem. 1959 Apr;234(4):967–972. [PubMed] [Google Scholar]
- Aldridge W. N., Rose M. S. The mechanism of oxidative phosphorylation A hypothesis derived from studies of trimethyltin and triethyltin compounds. FEBS Lett. 1969 Jul;4(2):61–68. doi: 10.1016/0014-5793(69)80197-3. [DOI] [PubMed] [Google Scholar]
- Aldridge W. N., Street B. W. Oxidative phosphorylation. Biochemical effects and properties of trialkyltins. Biochem J. 1964 May;91(2):287–297. doi: 10.1042/bj0910287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHANCE B., WILLIAMS G. R. Respiratory enzymes in oxidative phosphorylation. IV. The respiratory chain. J Biol Chem. 1955 Nov;217(1):429–438. [PubMed] [Google Scholar]
- Coleman J. O., Palmer J. M. The influence of pH on the inhibition of oxidative phosphorylation and electron transport by triethyltin. Biochim Biophys Acta. 1971 Sep 7;245(2):313–320. doi: 10.1016/0005-2728(71)90150-2. [DOI] [PubMed] [Google Scholar]
- Dawson A. P., Selwyn M. J. The action of trialkyltin compounds on mitochondrial respiration. The effect of pH. Biochem J. 1974 Mar;138(3):349–357. doi: 10.1042/bj1380349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris E. J., Bangham J. A., Zukovic B. Equilibration of chloride and pyruvate distributions between liver mitochondria and medium mediated by organo-tin salts. FEBS Lett. 1973 Feb 1;29(3):339–344. doi: 10.1016/0014-5793(73)80054-7. [DOI] [PubMed] [Google Scholar]
- Hind G., Jagendorf A. T. SEPARATION OF LIGHT AND DARK STAGES IN PHOTOPHOSPHORYLATION. Proc Natl Acad Sci U S A. 1963 May;49(5):715–722. doi: 10.1073/pnas.49.5.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hind G., Nakatani H. Y., Izawa S. The role of Cl- in photosynthesis. I. The Cl- requirement of electron transport. Biochim Biophys Acta. 1969 Feb 25;172(2):277–289. doi: 10.1016/0005-2728(69)90070-x. [DOI] [PubMed] [Google Scholar]
- Holland P. C., Clark M. G., Bloxham D. P., Lardy H. A. Mechanism of action of the hypoglycemic agent diphenyleneiodonium. J Biol Chem. 1973 Sep 10;248(17):6050–6056. [PubMed] [Google Scholar]
- Holland P. C., Sherratt H. S. Biochemical effects of the hypoglycaemic compound diphenyleneiodonnium. Catalysis of anion-hydroxyl ion exchange across the inner membrane of rat liver mitochondria and effects on oxygen uptake. Biochem J. 1972 Aug;129(1):39–54. doi: 10.1042/bj1290039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn J. S. Chlorotri-n-butylin. An inhibitor of photophosphorylation in isolated chloroplasts. Biochim Biophys Acta. 1968 Jan 15;153(1):203–210. doi: 10.1016/0005-2728(68)90161-8. [DOI] [PubMed] [Google Scholar]
- Kalberer P. P., Buchanan B. B., Arnon D. I. Rates of photosynthesis by isolated chloroplasts. Proc Natl Acad Sci U S A. 1967 Jun;57(6):1542–1549. doi: 10.1073/pnas.57.6.1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEE C. P., ERNSTER L. RESTORATION OF OXIDATIVE PHOSPHORYLATION IN NON-PHOSPHORYLATING SUBMITOCHONDRIAL PARTICLES BY OLIGOMYCIN. Biochem Biophys Res Commun. 1965 Feb 17;18:523–529. doi: 10.1016/0006-291x(65)90785-0. [DOI] [PubMed] [Google Scholar]
- Packer L., Deamer D. W., Crofts A. R. Conformational changes in chloroplasts. Brookhaven Symp Biol. 1966;19:281–302. [PubMed] [Google Scholar]
- Rose M. S., Aldridge W. N. Oxidative phosphorylation. The effect of anions on the inhibition by triethyltin of various mitochondrial functions, and the relationship between this inhibition and binding of triethyltin. Biochem J. 1972 Mar;127(1):51–59. doi: 10.1042/bj1270051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose M. S. Evidence for histidine in the triethyltin-binding site of rat haemoglobin. Biochem J. 1969 Jan;111(2):129–137. doi: 10.1042/bj1110129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuldiner S., Avron M. Anion permeability of chloroplasts. Eur J Biochem. 1971 Mar 11;19(2):227–231. doi: 10.1111/j.1432-1033.1971.tb01308.x. [DOI] [PubMed] [Google Scholar]
- Selwyn M. J., Dawson A. P., Stockdale M., Gains N. Chloride-hydroxide exchange across mitochondrial, erythrocyte and artificial lipid membranes mediated by trialkyl- and triphenyltin compounds. Eur J Biochem. 1970 May 1;14(1):120–126. doi: 10.1111/j.1432-1033.1970.tb00268.x. [DOI] [PubMed] [Google Scholar]
- Stockdale M., Dawson A. P., Selwyn M. J. Effects of trialkyltin and triphenyltin compounds on mitochondrial respiration. Eur J Biochem. 1970 Aug;15(2):342–351. doi: 10.1111/j.1432-1033.1970.tb01013.x. [DOI] [PubMed] [Google Scholar]
- Uribe E. G. Phloretin: an inhibitor of phosphate transfer and electron flow in spinach chloroplasts. Biochemistry. 1970 May 12;9(10):2100–2106. doi: 10.1021/bi00812a011. [DOI] [PubMed] [Google Scholar]
- Watling A. S., Selwyn M. J. Effect of some organometallic compounds on the permeability of chloroplast membranes. FEBS Lett. 1970 Oct 5;10(3):139–142. doi: 10.1016/0014-5793(70)80437-9. [DOI] [PubMed] [Google Scholar]
- West K. R., Wiskich J. T. Photosynthetic control by isolated pea chloroplasts. Biochem J. 1968 Oct;109(4):527–532. doi: 10.1042/bj1090527. [DOI] [PMC free article] [PubMed] [Google Scholar]


