Abstract
Immune checkpoint inhibitor (ICI) therapy is the new standard treatment for advanced or metastatic hepatocellular carcinoma (HCC); however, many patients still fail to respond. This study explored the expression and prognosis of programmed death ligand 1 (PD-L1), cluster of differentiation 24 (CD24), and cluster of differentiation 47 (CD47) in patients with hepatitis B virus-associated HCC (HBV-associated HCC). We analyzed sequencing data from the Cancer Genome Atlas (TCGA) and investigated the expression of PD-L1, CD24, and CD47 in HBV-associated HCC patients by immunohistochemistry and their relationship with prognosis and clinicopathological factors. HCC data from the TCGA database show that PD-L1 was substantially correlated with various immune cells. In 67 patients with HBV-associated HCC, high PD-L1 and CD24 expression levels were related to poor overall survival (OS) and progression-free survival (PFS). PD-L1 expression was significantly associated with the staging of HBV-associated HCC (p = 0.011) and Ki67 expression (p = 0.024). Correlation analysis between variables reveals that PD-L1 was significantly positively correlated with CD24 and CD47. High expression of PD-L1 and CD24 are risk factors for poor prognosis in HBV-associated HCC patients following curative resection. PD-L1 is significantly correlated with CD24 and CD47.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-83145-5.
Keywords: HBV-associated hepatocellular carcinoma, Immune checkpoint inhibitors, PD-L1, CD24, CD47
Subject terms: Cancer, Immunology, Gastroenterology, Medical research, Oncology
Introduction
Liver cancer is the fourth most common malignant tumor in China and the second leading cause of cancer-related death, after lung cancer. Approximately 90% of liver cancers are hepatocellular carcinoma (HCC)1,2. HCC is a typical inflammatory tumor, and one of the primary triggers of HCC is the chronic inflammation induced by hepatitis B virus (HBV) infection2. Despite the availability of effective vaccines and antiviral treatments for HBV, HBV-associated HCC remains one of the leading causes of death worldwide, especially in Asia and Africa3. Unfortunately, over 70% of HBV-associated HCC cases are diagnosed at an advanced stage, leading to limited treatment options and poor prognosis4. Prognostic indicators for post-operative HBV-associated HCC are still not fully understood.
As an immune-tolerant organ, the liver contains immune cells that make up 20% of the total liver cell population, creating a complex immune-tolerant microenvironment within the liver tissue5. Immune cells facilitate the growth of tumor cells throughout malignant tumorigenesis and progression6. Immune checkpoint inhibitors (ICIs), such as antibodies for programmed death 1 (PD-1) and programmed death ligand 1 (PD-L1), have become the hotspot of HCC immunotherapy7. The treatment paradigm for advanced HCC has shifted from monotherapy with tyrosine kinase inhibitors, exemplified by sorafenib, to a combination of targeted therapy and PD-1/PD-L1 inhibitors, known as “targeted-immunotherapy” combination treatment8. Although PD-1/PD-L1 inhibitors, representative of ICIs, have achieved breakthroughs as first- and second-line treatments of advanced liver cancer, they are limited by their efficacy rates of only approximately 10-30% and issues of low objective response rates and drug resistance9. Therefore, exploring new therapeutic targets and combination treatment strategies is necessary.
Cluster of differentiation 24 (CD24) serves as a costimulatory molecule for T lymphocyte responses and a regulator of autoimmunity10. CD24 drives the onset and development of tumors as an “oncogene,” promoting cell migration, invasion, and proliferation. It is a factor associated with poor prognosis in malignant tumors such as HCC10,11. Cluster of differentiation 47 (CD47) is a glycoprotein widely expressed in the immunoglobulin superfamily. High CD47 expression is observed in various human malignant tumors and is positively correlated with poor prognosis across different types of cancer12. This suggests that CD47 could be an attractive new target for cancer treatment. In recent years, the efficacy of anti-CD47 antibody therapy in treating various malignant tumors has garnered significant attention.
This study aims to explore the expression of PD-L1, CD24, and CD47 in HBV-associated HCC and their effect on patient prognosis.
Results
Identification and analysis of differentially expressed mRNAs of HCC
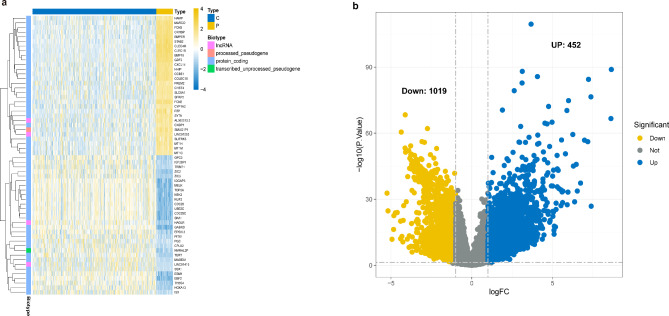
We first retrieved the transcriptome profiling data of patients with HCC from the Cancer Genome Atlas (TCGA) database. This study included 50 normal and 374 tumor samples. The data were analyzed using R software to study the differential expression of mRNAs. A total of 59,427 genes were identified; among which 1,471 were recognized as differentially expressed mRNAs, with 452 upregulated and 1,019 downregulated (Fig. 1).
Fig. 1.
Differentially expressed genes between HCC and adjacent (N = 424). (a). Heatmap demonstrating the differentially expressed genes between HCC and adjacent tissues. (b). Volcanoes indicate the 452 upregulated genes and 1019 down-regulated genes in HCC compared with adjacent tissues.
Enrichment analysis of signaling pathways and immune infiltration in HCC
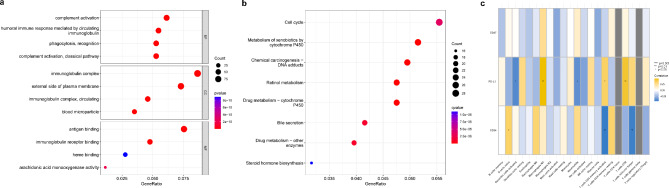
These differentially expressed genes were subjected to Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analyses. The GO enrichment results were concentrated in complement activation, immunoglobulin complex, and antigen binding. The KEGG enrichment results were focused on chemical carcinogenesis-DNA adducts and retinol metabolism. Correlation analysis was performed between essential genes and immune infiltration, revealing a significant correlation between PD-L1 and multiple immune cells (Fig. 2).
Fig. 2.
Enrichment and immune infiltration analysis in HCC. (a). GO enrichment analyses (N = 1471). (b). KEGG enrichment analyses (N = 1471). Pathway analysis was performed using the KEGG database (www.kegg.jp/kegg/kegg1.html). (c). Correlation analysis between key genes and immune infiltration (N = 424).
Prognostic of differentially expressed PD-L1, CD24, CD47 in HCC tissues
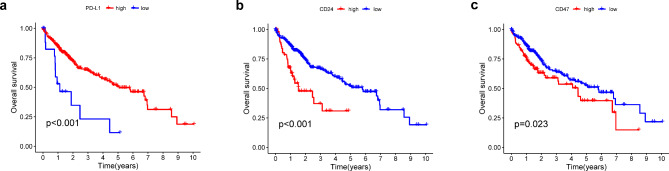
We studied the prognostic impact of differentially expressed PD-L1, CD24, and CD47 in the tumor tissues of patients with HCC whose data were sourced from the TCGA database. Kaplan-Meier survival analysis and log-rank test were used to compare the survival rates of patients in the high expression group (N = 188) and low expression group (N = 189). Kaplan-Meier analysis indicated that the patients exhibited different overall survival (OS) based on varying expression levels of PD-L1, CD24, and CD47 (Fig. 3).
Fig. 3.
Kaplan-Meier survival analysis comparing overall survival between the high-expression group (N = 188) and the low-expression group (N = 189) for PD-L1, CD24, and CD47. OS Overall survival.
Patient characteristics of HBV-associated HCC
Considering the limited data in the TCGA database, we attempted to evaluate the role of biomarkers in HBV-associated HCC using data from other patients. Sixty-seven patients with HBV-associated HCC were included; among them, 55 were male (82.09%), and 12 were female (17.91%), with an average age of 55.22 ± 10.81 years. All the patients received surgical treatment. The patients’ detailed characteristics are shown in Table 1.
Table 1.
Patient characteristics.
| Characteristics | Categorical variables | Characteristics | Continuous variables | |
|---|---|---|---|---|
| (percentage) | (mean ± standard) | |||
| Gender | Male | 55(82.09%) | Age (years) | 55.22 ± 10.81 |
| Female | 12(17.91%) | AFP (ng/mL) | 3192.06 ± 10962.96 | |
| Smoking | Yes | 48(71.64%) | CEA (ng/mL) | 2.91 ± 1.59 |
| No | 19(28.36%) | |||
| Drinking | Yes | 13(19.40%) | CA199 (U/mL) | 32.74 ± 38.64 |
| No | 54(80.60%) | |||
| HBeAg | Negative | 51(76.12%) | ALT (U/L) | 69.15 ± 85.77 |
| Positive | 16(23.88%) | |||
| Child-Pugh grade | A | 63(94.03%) | AST (U/L) | 63.49 ± 66.20 |
| B | 4(5.97%) | |||
| Number of tumors | 1 | 57(85.07%) | ALB (g/L) | 41.66 ± 5.18 |
| 2 | 7(10.45%) | TBIL (umol/L) | 14.33 ± 9.10 | |
| > 2 | 3(4.48%) | DBIL (umol/L) | 6.73 ± 5.76 | |
| Vascular invasion | No | 56(83.58%) | ||
| Yes | 11(16.42%) | IBIL (umol/L) | 7.60 ± 4.53 | |
| Invasion of the liver capsule | No | 13(19.40%) | RGT (µ/L) | 110.36 ± 138.92 |
| Yes | 54(80.60%) | AKP (U/L) | 107.49 ± 72.35 | |
| Histopathological grading | Low | 13(19.40%) | PT (sec) | 13.29 ± 1.40 |
| Intermediate | 49(73.13%) | WBC (×10^9/L) | 6.73 ± 2.77 | |
| High | 5(7.46%) | |||
| TNM stage | I | 33(49.25%) | PLT (×10^9/L) | 212.46 ± 155.53 |
| II | 22(32.84%) | |||
| III | 11(16.42%) | Hb (g/L) | 141.58 ± 17.33 | |
| IV | 1(1.49%) | |||
| Postoperative treatment modalities | RFA | 2(2.99%) | Maximum diameter of primary tumor (cm) | 7.16 ± 4.67 |
| TACE | 30(44.78%) | |||
| N/A | 35(52.24%) | |||
RFA radiofrequency ablation, TACE transcatheter arterial chemoembolization.
Survival analysis for HBV-associated HCC
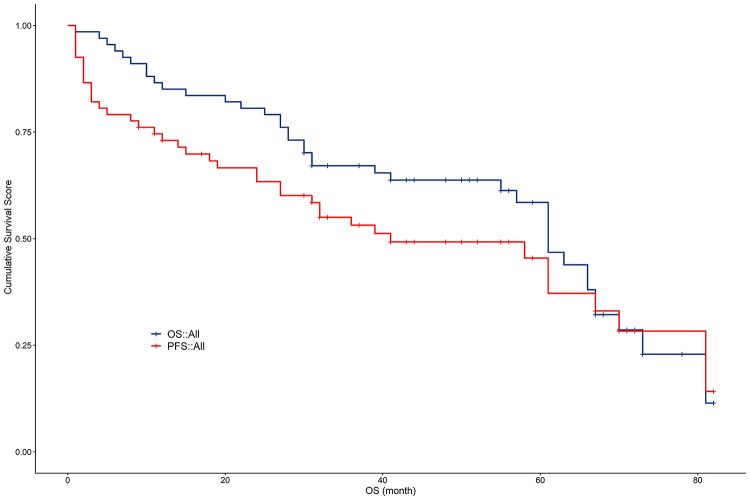
Among the 67 patients, the longest OS was 82 months, the shortest OS was 1 month, and the average OS was 43.24 ± 22.17 months. The longest progression-free survival (PFS) was 82 months, the shortest PFS was 1 month, and the average PFS was 32.32 ± 24.25 months. The median follow-up time was 44 months (95% CI: 38.692–49.308 months), the median OS was 61 months (95% CI: 55.067–66.933 months), and the median PFS was 41 months (95% CI: 20.535–61.465 months). The cumulative 1-year survival rate post-surgery was 86.57%, the 2-year survival rate was 80.60%, and the 5-year survival rate was 29.85%. The 1-, 2-, and 3-year progression-free rates post-surgery were 71.64%, 61.94%, and 16.42%, respectively (Fig. 4).
Fig. 4.
Survival Analysis Curve (N = 67). (a). OS survival curve. (b). PFS survival curve. OS overall survival, PFS progression-free survival.
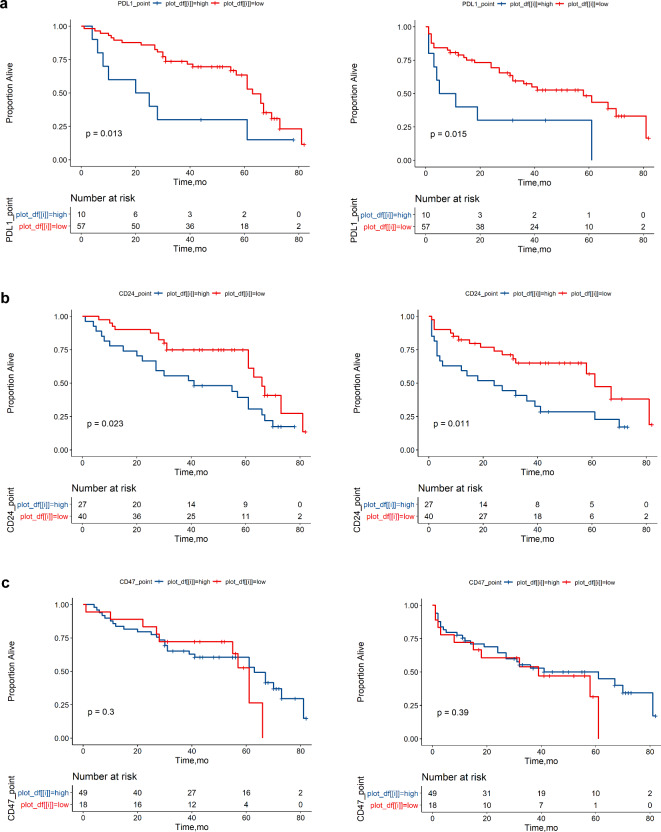
PD-L1, CD24, and CD47 in the prognostic analysis of HBV-associated HCC
Univariate survival analysis was conducted on PD-L1, CD24, and CD47 in relation to the OS and PFS of patients with liver cancer. For OS and PFS, the prognosis was significantly better in the low PD-L1 expression group than in the high expression group (OS, P = 0.013; PFS, P = 0.015). Similarly, the prognosis for the low CD24 expression group was better than that for the high expression group (OS, P = 0.023; PFS, P = 0.011). Analysis of prognosis between the high and low CD47 expression groups showed that the survival curves intersected between the groups, and no statistically significant difference was observed (OS, P = 0.3; PFS, P = 0.39) (Fig. 5).
Fig. 5.
The OS and PFS of HBV-associated HCC between high expression and low expression of PD-L1, CD24, and CD47 (N = 67). (a). PD-L1 OS survival curve and PFS survival curve. (b). CD24 OS survival curve and PFS survival curve. (c). CD47 OS survival curve and PFS survival curve. OS overall survival, PFS progression-free survival.
Analysis of prognostic factors in HBV-associated HCC
The prognostic factors influencing OS and PFS were analyzed using Cox univariate and multivariate regression models. Univariate analysis identified several factors significantly associated with OS and PFS, including smoking, TNM stage, number of tumors, PD-L1 expression, CD24 expression, and maximum diameter of the primary tumor (see Supplementary Table S1). Multivariate analysis further adjusted for confounding variables and revealed that smoking, maximum tumor diameter, and PD-L1 expression were independent predictors of OS and PFS (P < 0.05) (Table 2).
Table 2.
Multivariate analysis of prognostic factors in HBV-associated HCC.
| Factors | P (OS) | HR (OS) | 95%CI (OS) | P (PFS) | HR (PFS) | 95%CI (PFS) |
|---|---|---|---|---|---|---|
| Smoking | 0.04 | 2.062 | 1.035–4.11 | 0.014 | 2.433 | 1.201–4.931 |
| TNM stage | 0.665 | 0.177 | ||||
| Histopathological grading | 0.247 | 0.369 | ||||
| Number of tumors | 0.848 | 0.543 | ||||
| Maximum diameter of primary tumor(cm) | 0.008 | 1.091 | 1.023–1.163 | 0.008 | 1.089 | 1.023–1.159 |
| CD24 | 0.365 | 0.13 | ||||
| PD-L1 | 0.016 | 2.77 | 1.212–6.331 | 0.042 | 2.408 | 1.031–5.621 |
OS overall survival, PFS progression-free survival.
Relationship of PD-L1, CD24, and CD47 expression and clinicopathological factors
As shown in Table 3, PD-L1 expression was statistically associated with the staging of HBV-associated HCC (p = 0.003), tumor histopathological grading (p = 0.025), and Ki67 expression levels (p = 0.024). No statistically significant difference was found in tumor number, tumor size, cytokeratin 19, cytokeratin 18, cluster of differentiation 34 (CD34), glypican proteoglycan 3, and hepatocyte paraffin 1 (P > 0.05). Further Spearman correlation analysis revealed that PD-L1 was positively correlated with HCC staging and Ki67 expression. CD24 expression was significantly positively correlated with tumor staging (p = 0.048) but not with other clinicopathological factors (Table 4). In the analysis of CD47, statistical difference was found only with CD34, but the P-value was not statistically significant in the Spearman correlation coefficient analysis (P > 0.05) (see Supplementary Table S2).
Table 3.
Correlation between PD-L1 expression and clinicopathological factors in HBV-associated HCC.
| Factors | N | PD-L1 | P | Spearman correlation coefficient | |
|---|---|---|---|---|---|
| Low expression (n = 57) | High expression (n = 10) | ||||
| TNM stage (%) | 0.003 | 0.260* | |||
| I | 33 | 32(56.1) | 1 (10.0) | ||
| II | 22 | 18 (31.6) | 4 (40.0) | ||
| III | 11 | 7 (12.3) | 4 (40.0) | ||
| IV | 1 | 0 (0.0) | 1 (10.0) | ||
| Histopathological grading (%) | 0.025 | 0.214 | |||
| Low | 13 | 8 (14.0) | 5 (50.0) | ||
| Intermediate | 49 | 44 (77.2) | 5 (50.0) | ||
| High | 5 | 5 (8.8) | 0 (0.0) | ||
| Number of tumors (%) | 0.343 | ||||
| 1 | 57 | 50 (87.7) | 7 (70.0) | ||
| 2 | 7 | 5 (8.8) | 2 (20.0) | ||
| >2 | 3 | 2 (3.5) | 1 (10.0) | ||
|
Maximum diameter of primary tumor (cm) (mean ± SD) |
67 | 6.95 ± 4.32 | 8.34 ± 6.49 | 0.389 | |
|
CK19 (mean ± SD) |
67 | 0.46 ± 1.74 | 0.60 ± 1.265 | 0.804 | |
|
CK18 (mean ± SD) |
67 | 5.23 ± 4.09 | 4.10 ± 4.38 | 0.429 | |
|
CD34 (mean ± SD) |
67 | 2.74 ± 3.60 | 3.70 ± 3.95 | 0.444 | |
|
Glypican-3 (mean ± SD) |
67 | 3.77 ± 3.83 | 5.00 ± 4.37 | 0.363 | |
|
Hepar-1 (mean ± SD) |
67 | 2.39 ± 3.53 | 0.50 ± 1.27 | 0.102 | |
|
Ki67(%) (mean ± SD) |
67 | 24.91 ± 20.41 | 41.50 ± 21.56 | 0.024 | 0.262* |
‘*’indicates a significant correlation at a two-sided confidence level of 0.05.
Table 4.
Correlation between CD24 expression and clinicopathological factors in HBV-associated HCC.
| Factors | N | CD24 | P | Spearman correlation coefficient | |
|---|---|---|---|---|---|
| Low expression (n = 40) | High expression (n = 27) | ||||
| TNM stage (%) | 0.048 | 0.265* | |||
| I | 33 | 22 (55.0) | 11 (40.7) | ||
| II | 22 | 15 (37.5) | 7 (25.9) | ||
| III | 11 | 3 (7.5) | 8 (29.6) | ||
| IV | 1 | 0 (0.0) | 1 (3.7) | ||
| Histopathological grading (%) | 0.988 | ||||
| Low | 13 | 8 (20.0) | 5 (18.5) | ||
| Intermediate | 49 | 29 (72.5) | 20 (74.1) | ||
| High | 5 | 3 (7.5) | 2 (7.4) | ||
| Number of tumors (%) | 0.093 | ||||
| 1 | 57 | 36 (90.0) | 21 (77.8) | ||
| 2 | 7 | 4 (10.0) | 3 (11.1) | ||
| >2 | 3 | 0 (0.0) | 3 (11.1) | ||
|
Maximum diameter of primary tumor (cm) (mean ± SD) |
67 | 6.49 ± 4.20 | 8.14 ± 5.22 | 0.156 | |
|
CK19 (mean ± SD) |
67 | 0.40 ± 1.55 | 0.59 ± 1.87 | 0.647 | |
|
CK18 (mean ± SD) |
67 | 4.33 ± 4.12 | 6.15 ± 3.95 | 0.075 | |
|
CD34 (mean ± SD) |
67 | 2.25 ± 3.31 | 3.81 ± 3.95 | 0.084 | |
|
Glypican-3 (mean ± SD) |
67 | 3.93 ± 3.87 | 4.00 ± 4.03 | 0.939 | |
|
Hepar-1 (mean ± SD) |
67 | 2.57 ± 3.40 | 1.41 ± 3.24 | 0.164 | |
|
Ki67(%) (mean ± SD) |
67 | 26.68 ± 20.61 | 28.44 ± 23.25 | 0.744 | |
‘*’ indicates a significant correlation at a two-sided confidence level of 0.05.
Correlation analysis of PD-L1, CD24, and CD47
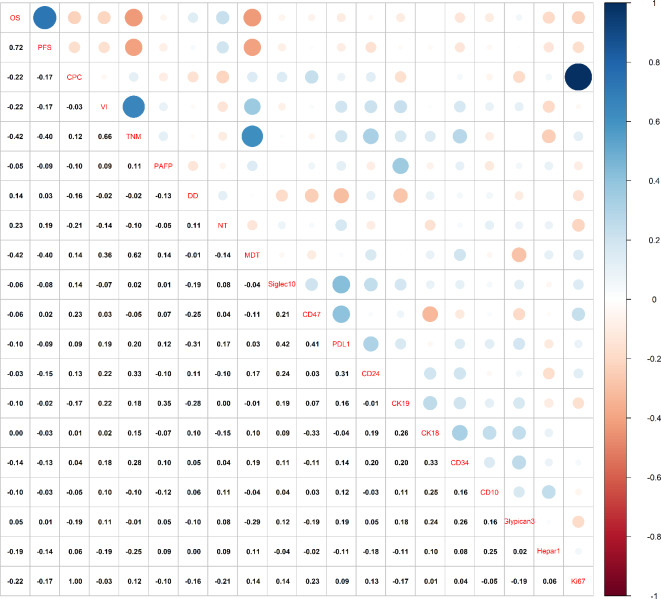
The results of correlation analysis between different variables, including PD-L1, CD24, and CD47 in patients with HBV-associated HCC, are shown in the scatter plot (Fig. 6). PD-L1 was positively correlated with CD24 and CD47 (r = 0.31, r = 0.41). CD47 was positively correlated with sialic acid-binding Ig-like lectin 10 (Siglec 10) and Child-Pugh grade (r = 0.21, r = 0.23). PD-L1 also showed positive correlations with Siglec 10 (r = 0.42), vascular invasion (r = 0.19), TNM staging (r = 0.20), and number of tumors (r = 0.17). Furthermore, CD24 was positively correlated with Siglec 10 (r = 0.24).
Fig. 6.
Correlation Analysis between Different Variables in HBV-associated HCC patients (N = 67). OS overall survival, PFS progression-free survival, CPC child-Pugh grade; VI vascular invasion, PAFP preoperative AFP, DD degree of differentiation, NT number of tumors, MDT maximum diameter of tumor, Siglec10 sialic acid-binding immunoglobulin-like lectin 10, Hepar1 hepatocyte paraffin 1.
Discussion
This study aimed to unravel the complex interplay among PD-L1, CD24, and CD47 in HBV-associated HCC and their collective impact on patient prognosis. The findings highlighted the elevated expression of CD24 and PD-L1 as potential harbingers of adverse outcomes in HBV-associated HCC, corroborating their roles in tumor progression and immune evasion mechanisms.
Although surgical resection is a potentially curative treatment option for HCC, the high recurrence rate of HCC post-surgery remains a significant threat and leads to low survival rates among patients, especially those with HBV-associated HCC13. PD-L1 expression is elevated in the liver tissues of chronic HBV carriers and the tumor tissues of patients with HBV-related HCC. This finding suggests that the PD-1/PD-L1 pathway may be involved in suppressing the host’s antiviral and antitumor immune responses, thereby facilitating the progression of HBV-associated HCC14,15. To protect themselves, tumor cells continuously express some immune checkpoint molecules at high levels on their surface, thereby inhibiting the function of T-cells16. This mechanism helps avoid attacks from immune cells, resulting in immune escape17. PD-L1 is one of these immune checkpoints. By binding to the PD-1 expressed in them, tumor cells can inhibit the function of immune cells and eventually evade detection18. However, current data on the prognostic role of PD-L1 in HCC are inconsistent19. Some studies found that PD-L1 does not have a significant prognostic effect on patients with liver cancer after therapeutic liver resection20. In the present work, the group with low PD-L1 expression showed a significantly better prognosis than the high expression group. Moreover, PD-L1 expression was significantly associated with the staging of HBV-associated HCC, tumor differentiation degree, and Ki67 expression. A correlation was found between PD-L1 and adverse clinicopathological factors in HBV-associated HCC, suggesting that PD-L1 can serve as a relevant risk factor in the prognosis evaluation of HBV-associated HCC.
The PD-1/PD-L1 pathway plays a crucial role in the immunotherapy of HCC, where inhibitors have achieved breakthroughs in treatment and offer hope to patients21,22. However, only a subset of patients respond to these treatments23. During chronic HBV infection, these PD1 + T cells become exhausted, leading to a reduced tumor response during anti-PD1 immunotherapy24. Furthermore, many responders develop resistance after an initial response, leading to tumor recurrence and treatment failure in patients with HCC25. Therefore, on the basis of current clinical outcomes, PD-1/PD-L1 antibodies may be suitable for combination therapy26. CD24 is overexpressed in various human malignancies, including in gastrointestinal tumors, HCC tissues, and highly metastatic HCC cell lines11,27,28. Kim et al.29 highlighted its role as a critical predictor for distant metastasis in patients undergoing curative surgery and subsequent adjuvant therapy, particularly in those with node-positive extrahepatic bile duct cancer. Yang et al.11 also revealed that CD24 predicts OS and PFS in HCC. The expression of CD24 is not associated with α-fetoprotein, tumor-node-metastasis staging, or Edmondson grading but is related to poor prognosis. Our study found that CD24 was significantly correlated with OS and PFS in HBV-associated HCC. It was also significantly related to the staging of HBV-associated HCC. This suggests that CD24 expression may have potential prognostic value in HBV-associated HCC and is associated with specific adverse clinical and pathological features. Moreover, the positive correlation between CD24 and PD-L1 suggests that combined targeted therapy against CD24 and PD-L1 antibodies could hold value in treating HBV-associated HCC.
The CD47/SIRPα axis was identified as the first tumor phagocytosis-related checkpoint, also known as the macrophage “do not eat me” signal30. Whether CD47 can serve as an independent risk factor for the prognosis of HCC remains uncertain. A recent study from South Korea analyzing survival outcomes based on CD47 expression in 166 HCC tissue samples found that CD47 expression was not related to PFS or OS in patients with HCC. However, CD47 expression was found to be related to PFS in patients with HCC who underwent surgical resection without any adjuvant treatment31. In our cohort, CD47 expression was not directly related to OS and PFS, indicating the multifaceted nature of immune evasion strategies adopted by tumor cells. This finding suggests that the impact of CD47 on patient prognosis could be modulated by other factors or signaling pathways within the tumor microenvironment. Its significant relationship with CD47 and PD-L1 expression implies a complex immune regulatory network. A combination therapy targeting CD47 and PD-L1 could be a novel and promising treatment strategy for HBV-associated HCC32.
Multivariate Cox regression analysis revealed smoking, maximum diameter of tumor (MDT), and PD-L1 expression as independent prognostic factors for HBV-associated HCC. Approximately 13% of HCC cases worldwide are associated with smoking, and its interaction with chronic viral hepatitis increases the risk of HCC33. HBV and hepatitis C virus primarily act as promoters through chronic inflammation, chronic hepatitis, and liver cirrhosis-induced cell proliferation. Introduced with the Milan criteria, a 5 cm MDT could serve as a threshold for liver transplant in cases of single HCC lesions, beyond which recurrence rates increase34. With the increase in MDT, the median serum AFP levels and the percentage of patients with portal vein thrombosis and multifocal lesions also increase, suggesting that the aggressiveness of liver cancer may worsen or evolve with tumor growth35. Therefore, intrinsic tumor characteristics and extrinsic factors, such as lifestyle habits, must be considered in the management and prognostic prediction of HCC. For treatment, targeted therapy must be integrated with lifestyle and environmental changes.
Our research also has some limitations. (1) This study involved 67 patients with HBV-associated HCC, which, while informative, represented a relatively small cohort. It also limited the generalizability of the findings across the broad population of patients with HBV-associated HCC, especially when considering the diverse genetic and environmental factors influencing the development and progression of this disease. (2) Our findings need to be validated in a randomized controlled trial. (3) The clinical efficacy of combining anti-CD24 and anti-CD47 with anti-PD1 must be evaluated, and (4) the complex immune characteristics of HBV-associated HCC require thorough elucidation.
Our study indicates that PD-L1 and CD24 can serve as predictors of poor prognosis in patients with HBV-associated HCC following curative resection, with PD-L1 identified as an independent prognostic risk factor. PD-L1 and CD24 expression levels are associated with certain adverse clinical pathological features of HBV-associated HCC. Moreover, PD-L1 is significantly positively correlated with CD24 and CD47, providing a basis for combined targeted therapy against immune checkpoints in clinical practice.
Materials and methods
Differential expression gene analysis of HCC and paracancerous tissues from the TCGA dataset
The Limma package (version 3.40.2) in R software was used to analyze the differential expression of mRNAs. Adjusted P-values were applied to correct for false positives in TCGA. A threshold of “Adjusted P-value < 0.05 and Log2 (fold change) > 1 or Log2 (fold change) < -1” was used to identify differentially expressed mRNAs. To confirm the underlying functions of potential targets, a functional enrichment analysis was performed. To investigate the carcinogenic roles of mRNAs, the ClusterProfiler package (version: 3.18.0) in R was employed to analyze GO terms and KEGG pathways.
Kaplan-meier analysis of gene features in the TCGA dataset using the survival and survminer packages in R
This study included 374 cases of HCC from the TCGA dataset. The overall survival times of differentially expressed PD-L1, CD24, and CD47 were compared using Kaplan-Meier analysis.
Immune infiltration analysis using CIBERSORT
CIBERSORT was used to analyze immune cell infiltration in HCC, with data processed using R version 4.0.3 (R Foundation for Statistical Computing, 2020) and visualized using the ggplot2 and pheatmap packages.
Patients and tumor samples
The study included 67 consecutive patients with HBV-associated HCC who underwent curative resection at the Fujian Provincial Hospital between January 2014 and January 2018. Patients included in the study were diagnosed with HCC based on postoperative pathology and tumor histological type, in accordance with the 2010 WHO classification criteria36. The specific inclusion criteria were as follows: (i) infection with HBV; (ii) pathological confirmation of HCC; (iii) liver function classified as Child-Pugh grade A or B; (iv) no prior anticancer treatment before surgery; (v) generally good overall health, without significant lesions in vital organs such as the heart, lungs, or kidneys. All methods and procedures in this study were conducted in accordance with Good Clinical Practice guidelines and the ethical principles of the Declaration of Helsinki. This study was approved by the Medical Ethics Committee of Fujian Provincial Hospital (Approval No. K2021-02-013), and informed consent was obtained from all patients.
Immunohistochemistry and quantification of PD-L1, CD24 and CD47 density
Formalin-fixed, paraffin-embedded Sect. (4 μm thick) from the tumor core and adjacent normal tissues of HBV-associated HCC were dewaxed and rehydrated. The sections were incubated overnight at 4 °C with rabbit SIGLEC10-C-terminal polyclonal antibody (ab198724; Abcam, USA), rabbit anti-human PD-L1 polyclonal antibody (ab58810; Abcam, USA), and SIRP-α1 polyclonal antibody (Cat# YT4301, China). Subsequently, the sections were serially washed and incubated with secondary antibodies. The stained sections were evaluated microscopically by pathologists using a double-blind method, ensuring they were unaware of the patients’ clinical data and outcomes. Staining intensity was scored as follows: 0 (negative), 1 (weak), 2 (moderate), and 3 (strong). The percentage of positive cells was scored as 0 (0%), 1 (1–10%), 2 (11–50%), and 3 (> 50%). The final score for each sample was calculated by multiplying the staining intensity score by the percentage score. Groups were defined as high expression when PD-L1 scores exceeded 3, CD24 scores exceeded 5, and CD47 scores were greater than 0; otherwise, they were classified as low expression. The staining intensity is shown in Supplementary Fig. S1–S3 online.
Follow-up and survival outcomes in patients with hbv-associated HCC
Postoperative follow-up was conducted through telephone interviews or outpatient visits. During the first two years, evaluations were performed every three months. From 2 to 5 years post-surgery, follow-ups were conducted every six months and annually thereafter. Patients’ survival status, serum AFP levels, and imaging results (abdominal CT and/or MRI) were recorded. OS was defined as the interval from surgery to death or the last follow-up, while PFS was defined as the time from surgery to tumor recurrence, death, or the last follow-up. The endpoint of follow-up was either death or the completion of the follow-up period.
Statistical analysis
Quantitative data were expressed as mean ± standard deviation (x ± s), while qualitative data were summarized as counts (n) and percentages (%). The survival package in R was used to determine the optimal cutoff points for PD-L1, CD24, and CD47 based on survival time. Samples were then categorized into high and low expression groups accordingly. Kaplan-Meier survival curves were generated to evaluate survival differences, and the log-rank test was applied for group comparisons. Correlations between these markers, clinicopathological parameters, and among variables were analyzed using SPSS 25.0 software, with the χ2 test, Fisher’s exact test, and T-test. A P-value < 0.05 was considered statistically significant.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Author contributions
All authors contributed to the study’s conception and design. H. X. and T. H. conceived, designed, and supervised the study. L. A. , W. M. and W. Z. performed the experiments, analyzed data, authored and reviewed the article. L. J. , L. Z. , L. S. , L. S. and L. H. collected data, prepared figures and tables. All authors reviewed and approved the final manuscript.
Funding
This research was funded by the Natural Science Foundation of Fujian Province (Grant number 2022J01121025), Joint Funds for the Innovation of Science and Technology, Fujian Province (Grant numbers 2023Y9327 and 2023Y9296).
Data availability
The data that support the plots within this paper and other findings of this study are available from the corresponding author upon reasonable request.
Declaration
Competing interests
The authors declare no competing interests.
Institutional review board statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Institutional Review Board of Fujian Provincial Hospital (Approval No. K2021-02-013).
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Aiping Lin, Mingxia Wang and Zhihui Wang contributed equally to this work.
Contributor Information
Haijun Tang, Email: tanghaijun16@163.com.
Xueping Huang, Email: hxuep@mail2.sysu.edu.cn.
References
- 1.Zheng, R. et al. Liver cancer incidence and mortality in China: Temporal trends and projections to 2030. Chin. J. Cancer Res.30, 571–579 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Villanueva, A. & Hepatocellular carcinoma N Engl. J. Med.380, 1450–1462 (2019). [DOI] [PubMed] [Google Scholar]
- 3.Bray, F. et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin.68, 394–424 (2018). [DOI] [PubMed] [Google Scholar]
- 4.Singal, A. G., Lampertico, P. & Nahon, P. Epidemiology and surveillance for hepatocellular carcinoma: New trends. J. Hepatol.72, 250–261 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gajewski, T. F., Schreiber, H. & Fu, Y. X. Innate and adaptive immune cells in the tumor microenvironment. Nat. Immunol.14, 1014–1022 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hinshaw, D. C. & Shevde, L. A. The tumor microenvironment innately modulates cancer progression. Cancer Res.79, 4557–4566 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abd, E. et al. Immune checkpoint inhibitors for unresectable hepatocellular carcinoma. Vaccines8, 616 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Llovet, J. M. et al. Immunotherapies for hepatocellular carcinoma. Nat. Rev. Clin. Oncol.19, 151–172 (2022). [DOI] [PubMed] [Google Scholar]
- 9.Sangro, B., Sarobe, P., Hervás-Stubbs, S. & Melero I. Advances in immunotherapy for hepatocellular carcinoma. Nat. Rev. Gastroenterol. Hepatol.18, 525–543 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Altevogt, P., Sammar, M., Hüser, L. & Kristiansen, G. Novel insights into the function of CD24: A driving force in cancer. Int. J. Cancer. 148, 546–559 (2021). [DOI] [PubMed] [Google Scholar]
- 11.Yang, X. R. et al. CD24 is a novel predictor for poor prognosis of hepatocellular carcinoma after surgery. Clin. Cancer Res.15, 5518–5527 (2009). [DOI] [PubMed] [Google Scholar]
- 12.Veillette, A. & Chen, J. SIRPα-CD47 immune checkpoint blockade in anticancer therapy. Trends Immunol.39, 173–184 (2018). [DOI] [PubMed] [Google Scholar]
- 13.Tung-Ping Poon, R., Fan, S. T. & Wong, J. Risk factors, prevention, and management of postoperative recurrence after resection of hepatocellular carcinoma. Ann. Surg.232, 10–24 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen, J. et al. Intrahepatic levels of PD-1/PD-L correlate with liver inflammation in chronic hepatitis B. Inflamm. Res.60, 47–53 (2011). [DOI] [PubMed] [Google Scholar]
- 15.Han, X. et al. Pre-treatment serum levels of soluble programmed cell death-ligand 1 predict prognosis in patients with hepatitis B-related hepatocellular carcinoma. J. Cancer Res. Clin. Oncol.145, 303–312 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hepatitis, B. virus X protein-mediated upregulation of miR-221 activates the CXCL12-CXCR4 axis to promote NKT cells in HBV-related hepatocellular carcinoma. Biocell 47, 1537–1548 (2023).
- 17.Liu, L. et al. Dynamic toxicity landscape of immunotherapy for solid tumors across treatment lines. J. Natl. Cancer Cent.3, 186–196 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Butte, M. J., Keir, M. E., Phamduy, T. B., Sharpe, A. H. & Freeman, G. J. Programmed death-1 ligand 1 interacts specifically with the B7-1 costimulatory molecule to inhibit T cell responses. Immunity27, 111–122 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang, B. et al. Advance in yumor immunotherapy: establishing a new paradigm for oncological treatment. Translational Surg. Oncol.1, 30–43 (2023). [Google Scholar]
- 20.Liu, G. M., Li, X. G. & Zhang, Y. M. Prognostic role of PD-L1 for HCC patients after potentially curative resection: a meta-analysis. Cancer Cell. Int.19, 22 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yi, M. et al. Combination strategies with PD-1/PD-L1 blockade: current advances and future directions. Mol. Cancer. 21, 28 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.A novel prognostic. target-gene signature and nomogram based on an integrated bioinformatics analysis in hepatocellular carcinoma. Biocell46, 1261–1288 (2022). [Google Scholar]
- 23.Kudo, M. Immune checkpoint inhibition in hepatocellular carcinoma: basics and ongoing clinical trials. Oncology92 (Suppl 1), 50–62 (2017). [DOI] [PubMed] [Google Scholar]
- 24.Lu, J. C. et al. Distinct PD-L1/PD1 Profiles and clinical implications in intrahepatic cholangiocarcinoma patients with different risk factors. Theranostics9, 4678–4687 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen, D. S. & Mellman, I. Oncology meets immunology: the cancer-immunity cycle. Immunity39, 1–10 (2013). [DOI] [PubMed] [Google Scholar]
- 26.Li, Q., Han, J., Yang, Y. & Chen, Y. PD-1/PD-L1 checkpoint inhibitors in advanced hepatocellular carcinoma immunotherapy. Front. Immunol.13, 1070961 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ni, Y. H., Zhao, X. & Wang, W. CD24, A review of its role in tumor diagnosis, progression and therapy. Curr. Gene Ther.20, 109–126 (2020). [DOI] [PubMed] [Google Scholar]
- 28.Yao, C. et al. Angiogenesis in hepatocellular carcinoma: mechanisms and anti-angiogenic therapies. Cancer Biology Med.20, 25 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim, H. J. et al. Different prognostic significance of CD24 and CD44 expression in breast cancer according to hormone receptor status. Breast20, 78–85 (2011). [DOI] [PubMed] [Google Scholar]
- 30.Oldenborg, P. A., Gresham, H. D. & Lindberg, F. P. Cd47-signal regulatory protein α (Sirpα) regulates Fcγ and complement receptor–mediated phagocytosis. J. Exp. Med.193, 855–862 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim, H., Bang, S., Jee, S., Paik, S. S. & Jang, K. Clinicopathological significance of CD47 expression in hepatocellular carcinoma. J. Clin. Pathol.74, 111–115 (2021). [DOI] [PubMed] [Google Scholar]
- 32.Dai, X. et al. Immunotherapy for targeting cancer stem cells in hepatocellular carcinoma. Theranostics11, 3489–3501 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chuang, S. C. et al. Interaction between cigarette smoking and hepatitis B and C virus infection on the risk of liver cancer: a meta-analysis. Cancer Epidemiol. Biomarkers Prev.19, 1261–1268 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mazzaferro, V. et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl. J. Med.334, 693–699 (1996). [DOI] [PubMed] [Google Scholar]
- 35.Carr, B. I. et al. Changes in hepatocellular carcinoma aggressiveness characteristics with an increase in tumor diameter. Int. J. Biol. Markers. 36, 54–61 (2021). [DOI] [PubMed] [Google Scholar]
- 36.Jf, F. [WHO Classification of digestive tumors: the fourth edition]. Ann. Pathol.31, (2011). [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the plots within this paper and other findings of this study are available from the corresponding author upon reasonable request.