Abstract
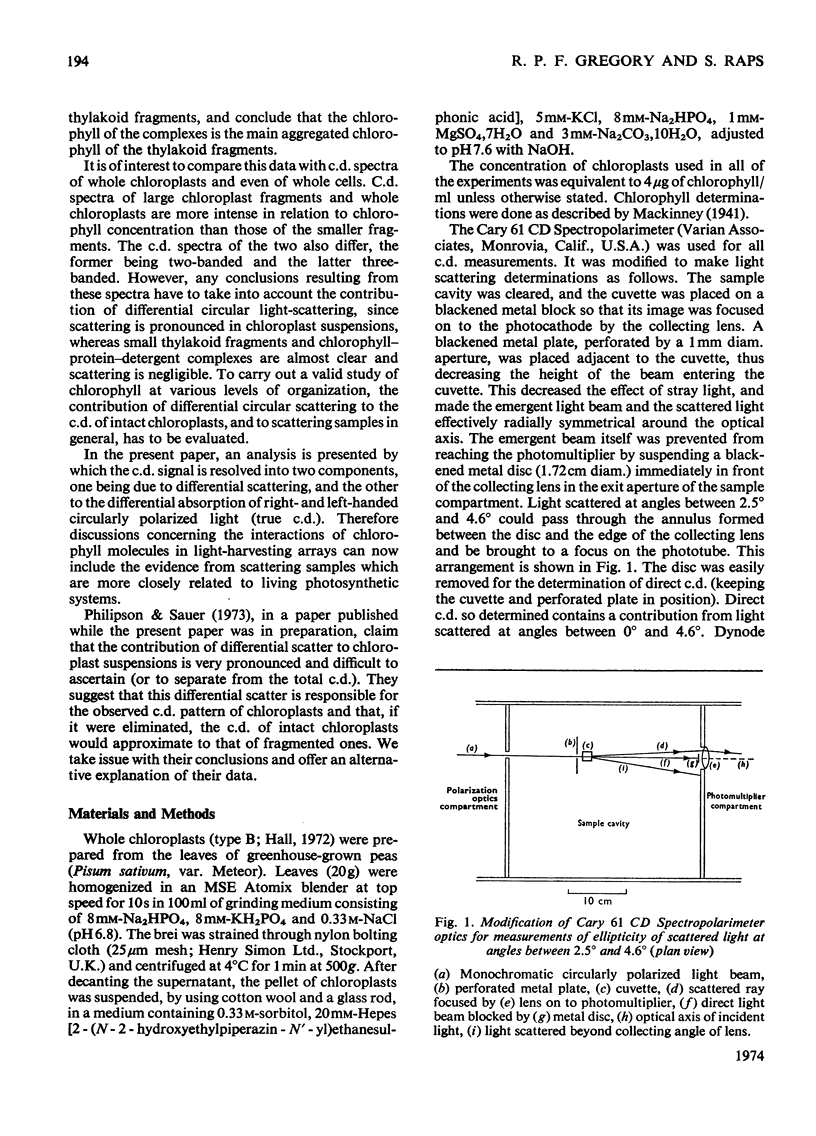
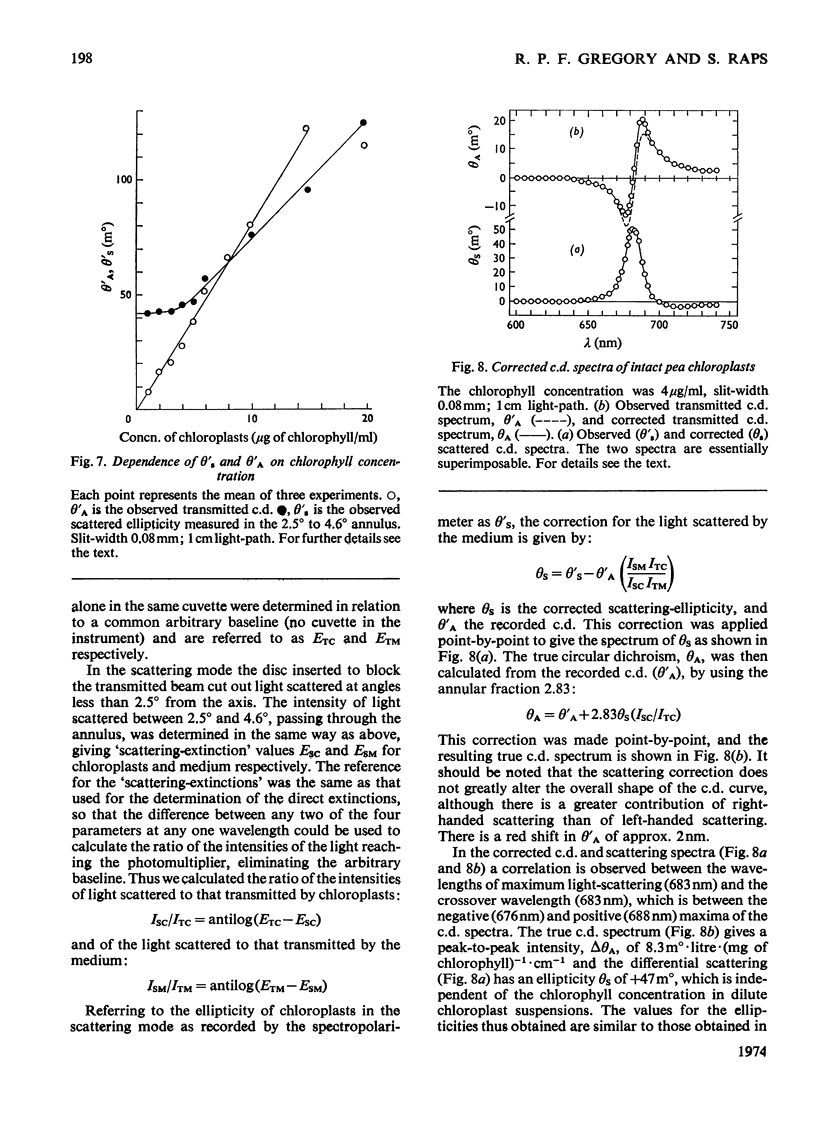
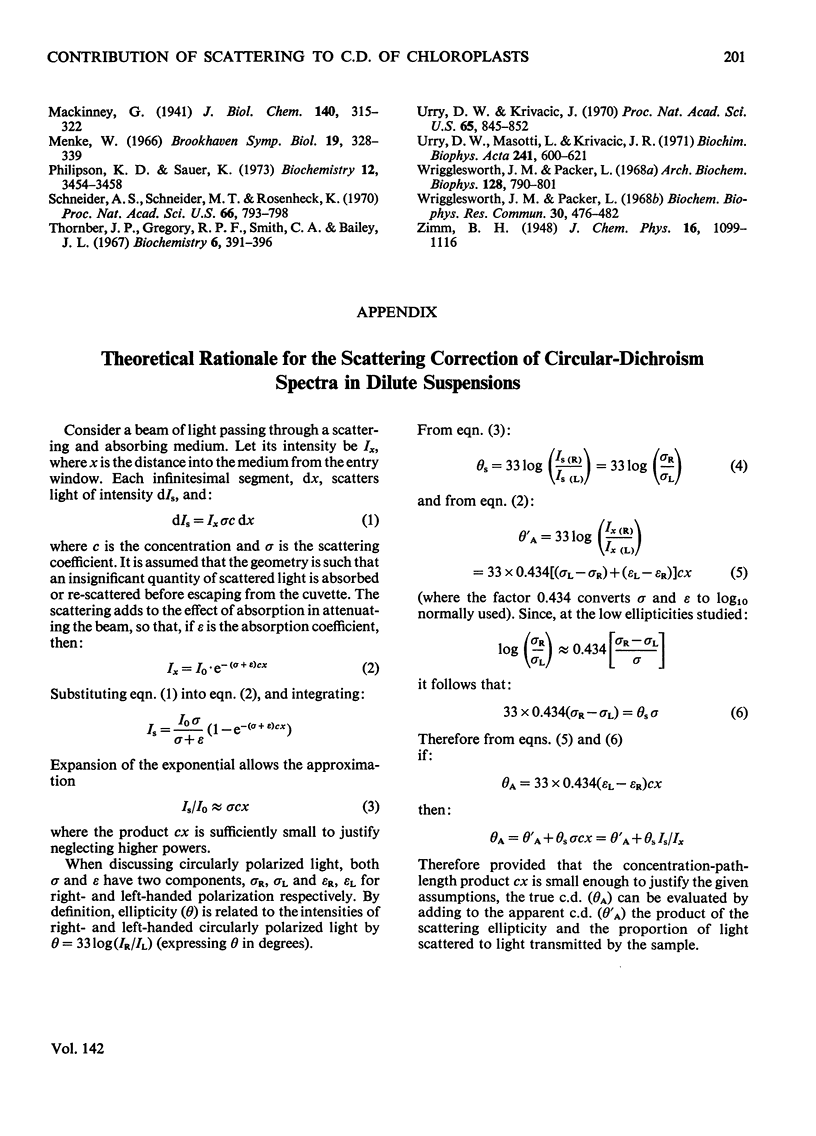
Chloroplasts isolated from pea leaves display an intense circular dichroism in the range 600 to 720nm. Circularly polarized light is also differentially scattered by chloroplasts, and this effect can be confused with circular dichroism. By using an instrumental modification it was possible to distinguish, and record separately, the ellipticities of the transmitted light (circular dichroism) and of the scattered light in the same c.d. instrument. By means of a light-scattering apparatus, the intensity of unpolarized light scattered by chloroplasts was measured as a function of wavelength and of angle. This measurement allowed the aforementioned ellipticities to be corrected for mutual interference. At a concentration of 4μg of chlorophyll/ml (the optimum practical concentration of chloroplasts at which there was no significant interaction of scattering and absorption effects) spectra of true circular dichroism (circular differential absorption) and circular differential scattering were obtained. The former showed maxima, positive at 688nm and negative at 676nm, with an intensity Δθ=8.3m°·litre·(mg of chlorophyll)−1·cm−1. The latter had a maximum at 683nm with an intensity of +47m° with respect to the solvent baseline; this value is independent of the concentration of chloroplasts in dilute suspensions. It is suggested that the intense circular dichroism of chloroplasts reflects specific chlorophyll–chlorophyll interactions in the light-harvesting pigment. The advantages of this method for determining the c.d. of scattering suspensions over those of other investigators are discussed.
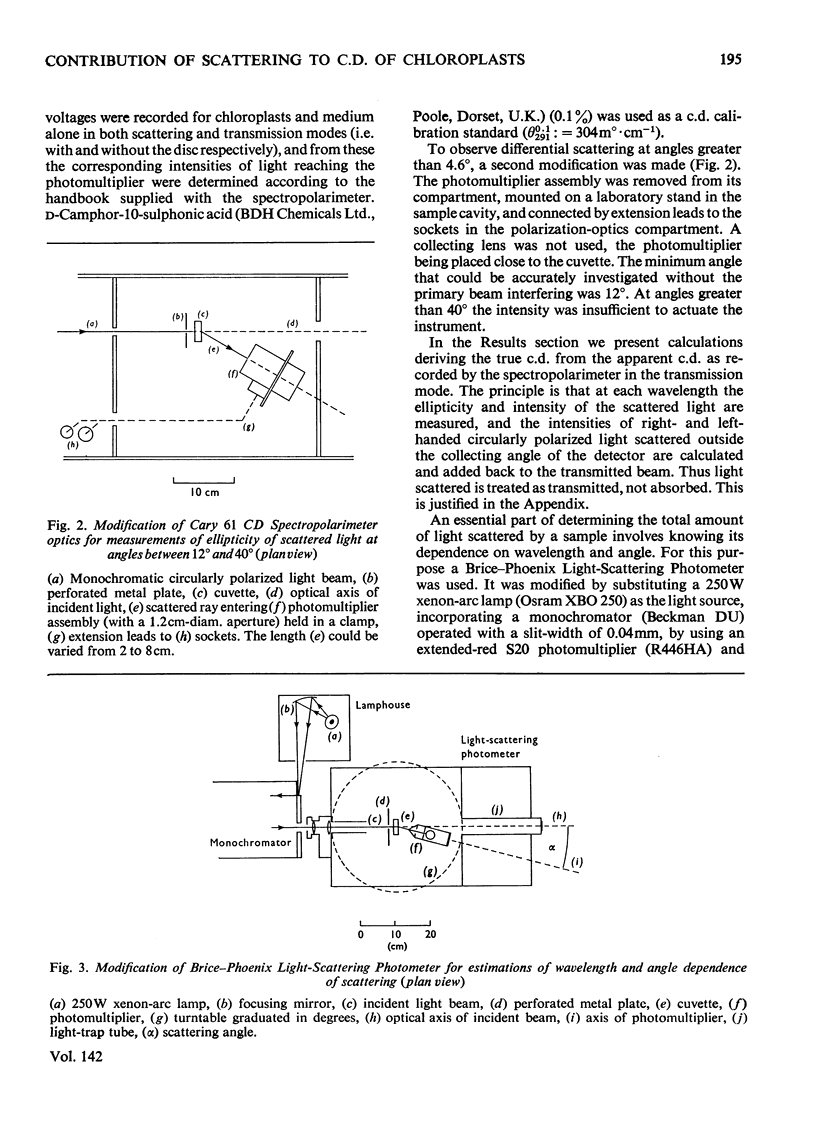
Full text
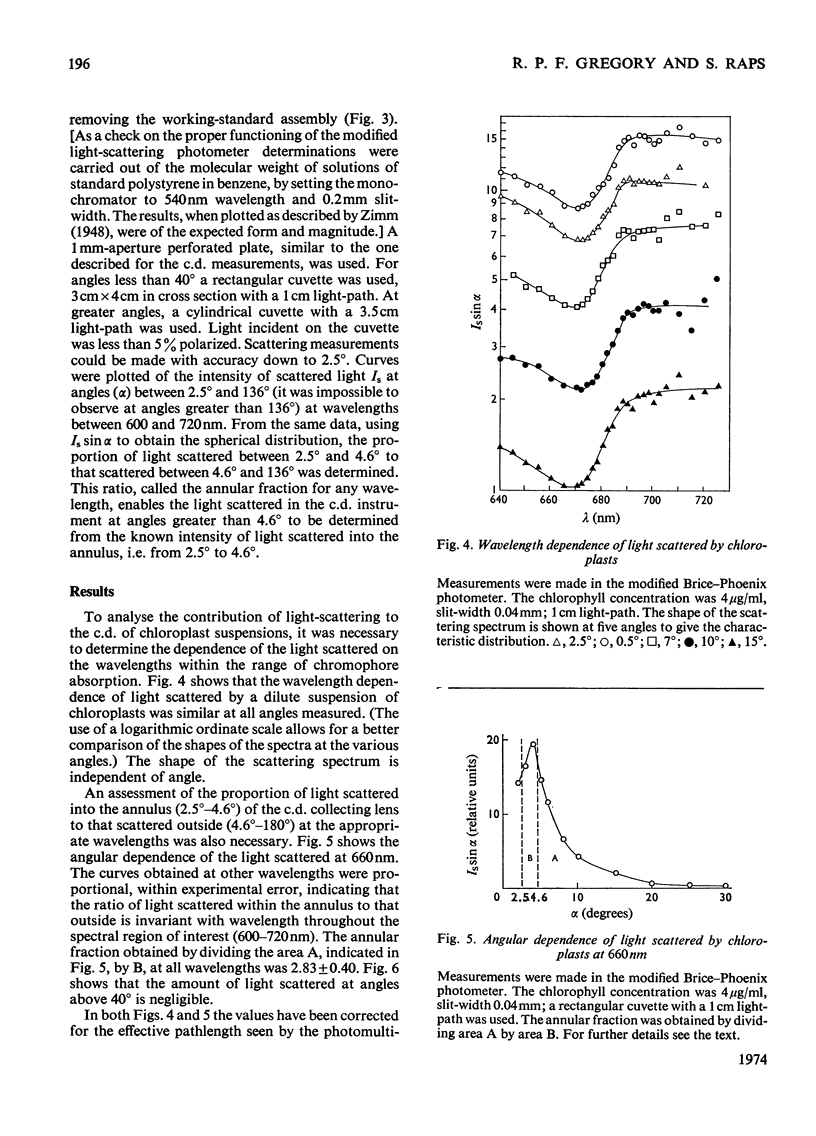
PDF
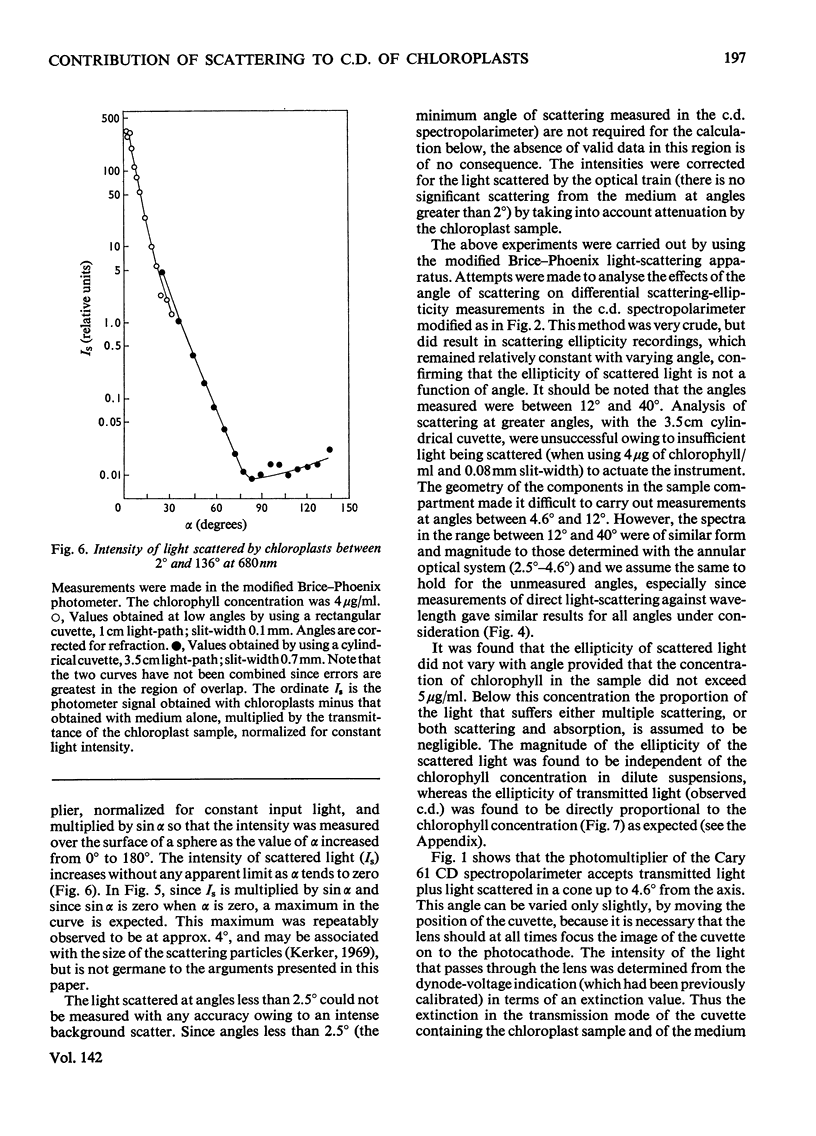


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- DUYSENS L. N. The flattening of the absorption spectrum of suspensions, as compared to that of solutions. Biochim Biophys Acta. 1956 Jan;19(1):1–12. doi: 10.1016/0006-3002(56)90380-8. [DOI] [PubMed] [Google Scholar]
- Dorman B. P., Maestre M. F. Experimental differential light-scattering correction to the circular dichroism of bacteriophage T2. Proc Natl Acad Sci U S A. 1973 Jan;70(1):255–259. doi: 10.1073/pnas.70.1.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dratz E. A., Schultz A. J., Sauer K. Chlorophyll-chlorophyll interactions. Brookhaven Symp Biol. 1966;19:303–318. [PubMed] [Google Scholar]
- Glaser M., Singer S. J. Circular dichroism and the conformations of membrane proteins. Studies with red blood cell membranes. Biochemistry. 1971 May 11;10(10):1780–1787. doi: 10.1021/bi00786a008. [DOI] [PubMed] [Google Scholar]
- Gordon D. J., Holzwarth G. Optical activity of membrane suspensions: calculation of artifacts by Mie scattering theory. Proc Natl Acad Sci U S A. 1971 Oct;68(10):2365–2369. doi: 10.1073/pnas.68.10.2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory R. P., Raps S., Bertsch W. Are specific chlorophyll-protein complexes required for photosynthesis? Biochim Biophys Acta. 1971 Jun 15;234(3):330–334. doi: 10.1016/0005-2728(71)90199-x. [DOI] [PubMed] [Google Scholar]
- Hall D. O. Nomenclature for isolated chloroplasts. Nat New Biol. 1972 Jan 26;235(56):125–126. doi: 10.1038/newbio235125a0. [DOI] [PubMed] [Google Scholar]
- Menke W. The molecular structure of photosynthetic lamellar systems. Brookhaven Symp Biol. 1966;19:328–340. [PubMed] [Google Scholar]
- Philipson K. D., Sauer K. Light-scattering effects on the circular dichroism of chloroplasts. Biochemistry. 1973 Aug 28;12(18):3454–3458. doi: 10.1021/bi00742a015. [DOI] [PubMed] [Google Scholar]
- Schneider A. S., Schneider M. J., Rosenheck K. Optical activity of biological membranes: scattering effects and protein conformation. Proc Natl Acad Sci U S A. 1970 Jul;66(3):793–798. doi: 10.1073/pnas.66.3.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornber J. P., Gregory R. P., Smith C. A., Bailey J. L. Studies on the nature of the chloroplast lamella. I. Preparation and some properties of two chlorophyll-protein complexes. Biochemistry. 1967 Feb;6(2):391–396. doi: 10.1021/bi00854a004. [DOI] [PubMed] [Google Scholar]
- Urry D. W., Krivacic J. Differential scatter of left and right circularly polarized light by optically active particulate systems. Proc Natl Acad Sci U S A. 1970 Apr;65(4):845–852. doi: 10.1073/pnas.65.4.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urry D. W., Masotti L., Krivacic J. R. Circular dichroism of biological membranes. I. Mitochondria and red blood cell ghosts. Biochim Biophys Acta. 1971 Aug 13;241(2):600–612. doi: 10.1016/0005-2736(71)90058-7. [DOI] [PubMed] [Google Scholar]
- Wrigglesworth J. M., Packer L. Optical rotary dispersion and circular dichroism studies on mitochondria: correlation of ultrastructure and metabolic state with molecular conformational changes. Arch Biochem Biophys. 1968 Dec;128(3):790–801. doi: 10.1016/0003-9861(68)90087-8. [DOI] [PubMed] [Google Scholar]
- Wrigglesworth J. M., Packer L. Optical rotation of mitochondria and its relation to conformation. Biochem Biophys Res Commun. 1968 Mar 12;30(5):476–482. doi: 10.1016/0006-291x(68)90076-4. [DOI] [PubMed] [Google Scholar]


