Abstract
Background:
Pomegranate (Punica granatum), fruit rich in bioactive constituents, is used as a feed supplement against bacterial pathogens in aquaculture.
Aim:
This study examined the effects of supplementing the diet of the common carp (Cyprino carpio) infected with Aeromonas veronii on growth and some hematological, biochemical, and immunological health indicators.
Methods:
Carp was fed for 7 weeks a diet of 30% crude protein and 7% crude fat, supplemented with 0, 0.5, 1.0, or 1.5% pomegranate peel, and growth was monitored. Hematological, biochemical, and immunological analyses were performed, including liver and antioxidant enzymes.
Results:
Bacteria from infected fish were identified by biochemical characteristics as A. veronii. Growth indicators (final body weight, weight gain, and specific growth rate), and feed utilization (relative growth rate and protein efficiency ratio) improved significantly in fish fed on 0.5% or 1.0% pomegranate-supplemented diets compared with the negative control (0%). red blood corpuscles, white blood cells, and Hct increased at all supplementation levels, and the highest hemoglobin was in the 1.5% group. Biochemical parameters, except globulin, decreased in fish-fed supplemented diets. No significant differences were observed in total protein and albumin levels. There was a significant improvement in immunological parameters and antioxidant enzymes.
Conclusion:
Dietary supplementation with pomegranate peel is a promising strategy for enhancing C. carpio’s health in the presence of A.veronii. Further work is necessary to determine the optimal supplementation level and its long-term effects.
Keywords: Pomegranate peel, Aquaculture, P. granatum, C. carpio, A. veronii
Introduction
Cyprinus carpio, commonly referred to as the common carp, is a globally important species in freshwater aquaculture. The Food and Agriculture Organization of the United Nations has documented that the worldwide cultivation of common carp exceeds 4.1 million metric tons annually, about 7.7% of the total global output from freshwater aquaculture operations (FAO, 2020; Chang et al., 2023). Intensive aquaculture practices have inadvertently amplified environmental stressors, thereby heightening the vulnerability of aquatic organisms to a range of pathogens, including viruses, bacteria, fungi, and parasites. Consequently, the incidence of infectious diseases among aquaculture species has increased (Nishida et al., 2018). To ensure the long-term viability of aquaculture in the face of production intensification, it is crucial to adopt strategies such as rigorous pathogen control, robust biosecurity protocols, and selective breeding for desirable traits (Abdelrahman et al., 2017; FAO, 2022). Intensified aquaculture practices can introduce management shortcomings that subject fish to a cascade of stressors (Balasch and Tort, 2019). These stressors include overcrowding, poor water quality, dietary deficiencies, nutritional imbalances, and physical disruptions during sorting and transport (Braun et al., 2010; Chen et al., 2022). Consequently, these compromised environmental and nutritional conditions negatively affect fish health and increase their susceptibility to disease outbreaks (Urbinati et al., 2020).
For decades, aquaculture has relied on the use of diverse chemoprophylactic agents such as antibiotics, hormones, vitamins, and therapeutic chemicals to mitigate stressors and maintain production efficiency (Boyd and Massaut, 1999). With the support of sustainable practices by the World Health Organization, researchers are now exploring natural food alternatives, such as edible plants. These alternatives offer advantages in cost-effectiveness, safety, and the potential to promote growth and immunity in aquaculture (Harikrishnan et al., 2011; Toutou et al., 2019). Food additives in the form of oil or powdered extract can be incorporated in the aquafeeds (Dawood et al., 2018; Abdel-Latif et al., 2020).
In the food and agricultural industries, a large amount of waste is generated, such as peels and seeds, which should be recycled without causing environmental hazards (RedCorn et al., 2018, Ghasemi et al., 2023). Punica granatum (pomegranate) is a plant with a well-documented history of therapeutic applications (Akhtar et al., 2015). Pomegranate peel represents about 35% of the fruit’s total weight (Ain et al., 2023) and is rich in bioactive compounds with antioxidant, antimicrobial, anti-inflammatory, and anti-carcinogenic properties, including anthocyanidins, hydroxybenzoic acids, hydrolysable tannins, and flavonoids (Al-Zoreky, 2009; Dahham et al., 2010; Rongai et al., 2015), as well as strong antimicrobial activity against Gram-positive bacteria (Radan et al., 2024). Due to its antimicrobial and antioxidant properties, pomegranate peel extract (PPE) has been added to minced poultry and rabbit meat (Forgione et al., 2024), as well as thornback ray (Raja clavata) sausages (Caglak et al., 2024), where it preserves lipid oxidation and maintains pH levels (Ghasemi et al., 2023). Moreover, P. granatum contains polyphenol compounds, such as ellagitannins, ellagic acid, and gallagic acid, which possess potent free radical scavenging activity (Akhtar et al., 2015; Vora et al., 2015).
The main bacterial diseases in fish farming include Vibrosis, Aeromonasis, Edwardsiellosis, Pseudomonasis, Flavobacteriosis, Mycobacteriosis, Streptococcosis, and Renibacteriosis, along with anaerobic bacteria such as Clostridium botulinum and Enterobacterium catenabacterium (Sahoo et al., 2020; Muniesa et al., 2020). The genus Aeromonasis comprises an array of Gram-negative, motile, anaerobic bacilli, which are ubiquitous in all aquatic environments and common in freshwater. Several Aeromonas species, including Aeromonas hydrophila, Aeromonas salmonicida, Aeromonas caviae, Aeromonas sobria, Aeromonas veronii, and Aeromonas jandaei, have caused severe morbidity and mortality in various fish populations (Medina-Morillo et al., 2023). Aeromonasis is the most common bacterial disease throughout the year in silver carp (Hypophthalmichthys molitrix) (Ali et al., 2014), grass carp (Ctenopharyngodon Idella) (Song et al., 2014), Indian carp (Labeo rohita) (Behera et al., 2023), and common carp (C. carpio) (Chang et al., 2023).
Some studies have suggested that PPE can enhance growth and combat various bacterial and viral diseases in aquaculture. For instance, Harikrishnan et al. (2012) observed the protective effect of PPE against the marine protozoan Philasterides dicentrarchi in olive flounder, while Acar et al. (2018) reported its effectiveness against the Gram-negative bacterium Yersinia ruckeri in rainbow trout. Similarly, Monir et al. (2020) and Gupta et al. (2023) noted the effects of PPE against A. hydrophila in Nile tilapia and rohu, respectively.
Given the widespread recommendations of many researchers for the use of PPE, this study investigated the effects of PPE as a food additive for common carp infected by A. veronii, focusing on growth performance, hematological and biochemical parameters, antioxidant and liver enzymes, and immunological activity.
Material and Methods
Fish
Common carp (average 10.5 ± 1.4 g) was obtained from the aquaculture unit of Marine Science Center, University of Basra. The fish exhibited clinical symptoms of infection with bacteria, which were isolated and biochemically identified. A total of 60 fish were randomly distributed into 12 60-l tanks filled with tap water. Each tank was equipped with a 24-hour air pump and kept under a natural photoperiod. The water conditions were as follows: temperature 23.4°C–24.3°C, salinity 1.3–1.8 psu, pH 8.4–8.8, and dissolved oxygen 7.2–7.6 mg l. The water was renewed twice a week. Fish were acclimated for 2 weeks before the experiment and were fed a commercial diet twice daily (at 08:00 am and 05:00 pm) at a feeding rate of 4% body weight.
Biochemical analysis of pathogenic bacteria
Gram staining was used to observe the morphological characteristics of unknown bacteria isolates. The biochemical characteristics, including maltose, glucose, sucrose, lactose, fructose, raffinose, xylose, melezitose, sorbose, L-rhamnose, sorbitol, inositol, dulcitol, adonitol, urea, esculin, Voges-Proskauer test, tartrate, malonate, citrate, starch, salicin, H2S, indole, lysine decarboxylase, ornithine decarboxylase, arginine dihydrolase, and motility were measured with a commercial micro-test system (Hangzhou Binhe Microorganism Reagent Co. Ltd., China) according to the manufacturer’s instructions.
Pomegranate peel extract
Pomegranate (P. granatum) fruits were procured from local markets in Qalat Sukar, Thi-Qar Governorate, Iraq, in May 2022. They were thoroughly washed with sterile distilled water and shade-dried. The dried material was ground into a powder and stored at –25°C. Pomegranate extract was produced from the powder according to Ismail et al. (2012). The extract was transferred to clean, opaque glass bottles and stored at 4°C for further use.
Fish diets and feeding
Experimental diets were formulated to be isonitrogenous (30% crude protein and 7% lipid). The detailed compositions and proximate analyses of these diets are presented in Table 1. Formulations adhered to the established nutritional requirements for fish, as outlined by the National Research Council (NRC, 1993). A basal control diet was created to meet these requirements, providing 30.23% crude protein and 4,362 kcal kg-1 gross energy. All dry ingredients and extracts were thoroughly mixed to homogeneity.
Table 1. Constituents and proximate composition (%) of the experimental basal diet.
| Constituents | PPE diet types | |||
|---|---|---|---|---|
| PPE (%) | 0 | 0.5 | 1.0 | 1.5 |
| Fish meal(g/100 g) | 40.2 | 40.2 | 40.2 | 40.2 |
| Soybean meal(g/100 g) | 30 | 30 | 30 | 30 |
| Yellow starch(g/100 g) | 7 | 7 | 7 | 7 |
| Wheat bran(g/100 g) | 6.8 | 6.8 | 6.8 | 6.8 |
| Fish oil(g/100 g) | 13 | 12.5 | 12 | 11.5 |
| Vitamins and Minerals1(g/100 g) | 3 | 3 | 3 | 3 |
| Gross composition (% DM) | ||||
| Protein | 30.22 | 30.11 | 30.17 | 30.09 |
| Lipid | 6.78 | 6.89 | 6.8 | 6.9 |
| Ash | 12.11 | 12.76 | 12.32 | 12.32 |
| Fiber | 5.59 | 5.61 | 5.86 | 5.67 |
| N.F.E. (mg/100 g) 2 | 50.89 | 50.24 | 50.71 | 50.69 |
| M.E. (kcal/100 g) 3 | 368.51 | 366.64 | 367.82 | 368.20 |
| Lysine | 1.81 | 1.88 | 1.79 | 1.80 |
| Methionine | 0.48 | 0.46 | 0.45 | 0.44 |
| Threonine | 1.26 | 1.22 | 1.24 | 1.25 |
1 Vitamins and minerals (A, D3, B1, B2, B6, B12, B3, B5, C, E, H, B9, Calcium, Cobalt, Magnesium, FeIron, Copper, Zinc, Potassium, Manganese, Choline Chloride). 2 Nitrogen-free extract (calculated by difference) = 100 - (protein + lipid + ash). 3Metabolizable energy (M.E.) was calculated as 4.5, 8.1 and 3.49 kcal/100 g for protein, lipid and N.F.E., respectively, according to (Pantha, 1982).
Subsequently, 100 ml of water per kilogram of diet was added, and the mixture was blended into a paste in a blender. Pellets were formed by extruding the paste through a laboratory pellet machine fitted with a 1-mm diameter die. The pellets were dried in a forced-air oven (Fisher Oven 13-261-28A) at 65°C for 24 hours and stored in plastic bags at 4°C throughout the experiment.
To assess the effects of PPE, the basal diet was supplemented with PPE at concentrations of 0.5%, 1.0%, and 1.5% (w/w). Each treatment was performed in triplicate, with five fish per replicate (a total of 60 fish). Fish were fed twice daily at a rate of 3% of their body weight for 7 weeks. The amount of feed was adjusted bi-weekly to account for changes in fish biomass. The proximate composition (moisture, crude protein, fat, and crude fiber) of the feedstuffs used in diet formulation was determined according to the methods outlined by AOAC (2005).
Growth performance parameters
Fish were weighed biweekly to determine appropriate feed intake (FI) and calculations were done using the following equations:
Weight gain (WG) = W2 − W1 (g)
Specific growth rate (SGR%) = 100(ln W2 − ln W1)/T
Relative growth rate (RGR%) = W2 − W1 (g)/W1 (g) × 100
Feed conversion ratio (FCR) = FI /BWG (g)
Protein efficiency ratio (PER) = BWG (g)/protein intake (g)
where W1 is the initial body weight (g), W2 is the final body weight (g), and T is the number of days in the feeding period.
Hematological and biochemical analyses
For serum preparation, whole blood samples were collected from the heart in tubes without anticoagulant and clotted blood was centrifuged at 3,000 rpm for 15 minutes. The collected serum was stored at –20°C for subsequent chemical and immunohistochemical analyses. Blood samples containing anticoagulants were used for analysis of hemoglobin concentration (HGB) and counts of red blood corpuscles (RBC) and white blood cells (WBC) as described by Natt and Herrick (1952). Hematocrit (Hct) was measured with an Auto Counter instrument (Decie and Lewis, 2006). The concentrations of total protein (TP) and albumin (Alb) were determined using colorimetric methods following established protocols outlined by Tietz (1990) and Wotton and Freeman (1982), respectively. The globulin concentration was then derived by subtracting the measured Alb concentration from the TP concentration.
Liver enzymes
Liver enzyme activities were measured following the methods described by Bergmeyer et al. (1976). Aspartate aminotransferase (AST) activity was determined using an Erba enzymatic assay kit (Erba Diagnostics, Mannheim, Germany). Briefly, 500 µl of R1 reagent was pre-incubated at 37°C in a test tube and 50 µl of the sample was added. The reaction was monitored continuously for 3 minutes at 37°C using an autoanalyzer. Alanine aminotransferase (ALT) activity was measured similarly using an Erba enzymatic assay kit (Erba Diagnostics), incubating the R1 reagent at 37°C before adding the sample. The reaction was continuously monitored for the specified time using the autoanalyzer. Alkaline phosphatase (ALP) activity was quantified using a MERCK enzymatic assay kit (Merck KGaA, Darmstadt, Germany). R1 reagent (400 µl) and R2 reagent (100 µl) were mixed and pre-incubated at 37°C for 1 minute. Then, 10 µl of the sample was added, and the reaction was monitored for 4 minutes at 37°C using the auto analyzer. All enzyme activities are expressed in international units per liter (IU/l). To minimize experimental errors and ensure data reproducibility, all assays were performed in triplicate.
Immunological activity
Lysozyme activity in serum was measured using a turbidimetric assay as described by Nudo and Catap (2011). A volume of 25 µl of each serum sample was incubated with 175 µl of Micrococcus luteus suspension (0.75 mg/ml) prepared in phosphate-citrate buffer (Sigma, ATCC 4698) at 25°C for 30 minutes. The optical density (OD) of the mixture was measured at 530 nm in a plate reader (Thermo Multiskan Go). The assay was calibrated with hen egg white lysozyme to convert the observed reduction rate in sample OD to lysozyme concentration (mg/ml). Total myeloperoxidase (MPO) content was measured following a modified protocol from Acar et al. (2018). Briefly, 10 µl of serum was diluted with 90 µl of Hank’s Balanced Salt Solution lacking calcium and magnesium ions in a 96-well plate. Then, 35 µl of a solution containing 0.1 mg/ml of 3,30,5,50-tetramethylbenzidine dihydrochloride and 0.006% hydrogen peroxide were added to each well. After incubation for 2 minutes, the reaction was terminated by adding 35 µl of 4 M sulfuric acid. The final optical density was measured at 450 nm using a plate reader.
Antioxidant enzymes
The activity of superoxide dismutase (SOD), a crucial antioxidant enzyme that neutralizes superoxide radicals, was quantified using the method described by Nishikimi et al. (1972). Additionally, catalase (CAT) activity, essential for breaking down hydrogen peroxide, was measured based on the protocol by Aebi (1974).
Statistical analysis
Data were processed and analyzed in IBM SPSS version 22. Results are presented as mean values ± standard deviation. Intergroup comparisons between the control group and each experimental group (or between groups) were done with a least significant difference post-hoc test to identify statistically significant differences. One-way ANOVA was used to determine statistical significance (p-value ≤ 0.05).
Ethical approval
Not needed for this study.
Results
The biochemical properties in Table 2 support the identification of the bacterial species as Aeromonas veronii. The isolate’s motility and its capacity to hydrolyze urea are the main criteria. The isolate showed negative findings for raffinose, xylose, melezitose, sorbose, L-rhamnose, sorbitol, inositol, dulcitol, and adonitol, but positive results for the Voges-Proskauer test, malonate, and acid generation from maltose, arabitol, mannose, glucose, sucrose, lactose, and fructose. The morphological, physiological, and biochemical characteristics of the isolated bacteria identified it as A. veronii, a Gram-negative, rod-shaped bacterium.
Table 2. Biochemical characteristics of the bacterial isolate.
| Characteristics | Reaction | Characteristics | Reaction |
|---|---|---|---|
| Acid formation from | Production of | ||
| Maltose | + | H2S | − |
| Arabitol | + | Indole | + |
| Mannose | + | Lysine decarboxylase | + |
| Glucose | + | Ornithine decarboxylase | + |
| Sucrose | + | Arginine dihydrolase | − |
| Lactose | + | Growth on | |
| Fructose | + | at 0% of NaCl | + |
| Raffinose | − | at 1% of NaCl | + |
| Xylose | − | at 2% of NaCl | + |
| Melezitose | − | at 3% of NaCl | + |
| Sorbose | − | at 4% of NaCl | + |
| L-rhamnose | − | at 5% of NaCl | − |
| Sorbitol | − | 4°C | + |
| Inositol | − | Motility | + |
| Dulcitol | − | Hemolytic | + |
| Adonitol | − | Utilization of | |
| Hydrolysis of | Citrate | − | |
| Esculin | − | Malonate | + |
| Urea | + | Salicin | − |
| Voges-Proskauer test | + | Starch | − |
| Tartrate | − | ||
(+): positive; (−): negative.
Experiments on the effects of PPE-supplemented diet on the growth and feed utilization of common carp showed no statistically significant differences in initial body weight (IBW) among the groups. The results in Table 3 show that supplementation of feed with 0.5% or 1.0% PPE significantly increased final body weight (FBW), weight gain (WG), specific growth rate (SGR), and relative growth rate (RGR) compared to the control group (0% PPE) (p < 0.05). However, there was no significant difference in growth performance between the 1.5% PPE group and the control group, implying that the optimal concentration is below 1.5%.
Table 3. Growth performance parameters of common carp (C. carpio) and their utilization of feed supplemented with different concentrations of PPE.
| Parameter/group | Control (0%) | PPE in diets | ||
|---|---|---|---|---|
| 0.5% | 1.0% | 1.5% | ||
| IBW | 10.67 ± 0.12a | 10.76 ± 0.09 | 10.87 ± 0.13 | 10.55 ± 0.32 |
| FBW | 25.27 ± 0.27b | 30.45 ± 0.81a | 30.80 ± 0.41a | 26.55 ± 0.75b |
| WG | 14.59 ± 0.38b | 19.68 ± 1.02a | 19.92 ± 0.61a | 16.0 ± 1.27b |
| SGR | 1.23 ± 0.29b | 1.48 ± 0.51a | 1.49 ± 0.40a | 1.31 ± 0.98b |
| RGR | 136.75 ± 4.91b | 182.93 ± 10.23a | 183.24 ± 7.92a | 151.98 ± 17.74b |
| FCR | 2.45 ± 0.58b | 1.58 ± 0.78a | 1.91 ± 0.63ab | 2.64 ± 0.54ab |
| PER | 0.48 ± 0.01b | 0.65 ± 0.02a | 0.66 ± 0.01a | 0.53 ± 0.03b |
Means values (±) in the same column sharing the same superscript are not significantly different. Absence of letters indicates no significant difference (ANOVA, one-way, p ≥ 0.05). IBW = Initial body weight (gm), FBW = Final body weight (gm), WG= Weight gain (gm), SGR= Specific growth rate (%/day), RGR=Relative growth rate (%), FCR= Feed conversion ratio, PER=Protein efficiency ratio (%).
The 0.5% ratio achieved the best value for feed conversion ratio (FCR) compared to the control group (1.58% and 2.45% for 0.5% and 0% PPE, respectively; p < 0.05). The results of PER did not differ from those obtained for FBW, WG, SGR, and RGR, as there was no significant difference between 1.5 and the control, while the best values were for the two lower concentrations (0.5% and 1%).
Hematological and biochemical results are shown in Table 4. RBC counts were significantly higher (p < 0.05) in all PPE groups (0.5%, 1.0%, and 1.5%) than in the control. Like RBC count, Hct levels increased significantly (p < 0.05) with increasing PPE supplementation compared to the control group (p < 0.05). WBC count showed a dose-dependent increase with increasing levels of PPE. The highest and middle supplementation levels (1.5% and 1.0%) resulted in significantly higher WBC counts compared to the control group (p < 0.05), while 0.5% PPE resulted in an intermediate WBC level that differed significantly (p < 0.05) from both the 1.5% and 0% PPE supplementation levels. HGB showed no statistically significant difference between the control (0% PPE) and supplemented groups (0.5%, 1.0%, 1.5% PPE).
Table 4. Hematological and biochemical parameters of common carp, C. carpio, fed a diet supplemented with different concentrations of PPE.
| Parameter/group | PPE in diets | |||
|---|---|---|---|---|
| Control (0%) | 0.5% | 1.0% | 1.5% | |
| Hematological parameters | ||||
| RBC | 1.33 ± 0.02b | 1.52 ± 0.06a | 1.59 ± 0.05a | 1.54 ± 0.06a |
| WBC | 2.44 ± 0.04c | 3.24 ± 0.49b | 3.82 ± 0.04ab | 3.94 ± 0.05a |
| Hct | 28.93 ± 1.06b | 29.19 ± 0.78a | 29.48 ± 0.73a | 29.30 ± 0.83a |
| HGB | 2.45 ± 0.05ab | 2.49 ± 0.35ab | 2.26 ± 0.14b | 3.35 ± 0.83a |
| Biochemical parameters | ||||
| CHOL | 8.58 ± 1.25a | 5.77 ± 0.32b | 5.99 ± 0.94b | 5.69 ± 0.24b |
| Glo | 27.45 ± 0.27b | 29.52 ± 0.45a | 30.96 ± 0.59a | 31.73 ± 0.75a |
| TP | 33.89 ± 1.19 | 35.07 ± 0.67 | 35.41 ± 0.94 | 35.50 ± 0.49 |
| Alb | 5.77 ± 0.34 | 5.55 ± 0.38 | 5.45 ± 0.33 | 5.43 ± 0.16 |
| ALP | 55.64 ± 2.94a | 46.02 ± 1.71b | 40.87 ± 5.30bc | 35.11 ± 3.38c |
| AST | 173.71 ± 2.31a | 151.60 ± 1.02a | 143.01 1.89b | 141.43 ± 5.19b |
| ALT | 27.89 ± 2.68a | 14.14 ± 1.79b | 14.32 ± 1.41b | 14.33 ± 2.21b |
Means values (±) in the same column sharing the same subscript are not significantly different, absence of letters indicates no significant difference (ANOVA, one-way, p ≥ 0.05). RBC= Red blood cell (×106 µl-1), WBC= White blood cell (×106 µl-1), Hct= Hematocrit (%), HGB= Hemoglobin (mmol L-1), CHOL= Cholesterol (mmol L-1), Glo = Globulin (g L-1), TP= Total Protein (g L-1), Alb= Albumin (g L-1), ALP= Alkaline phosphatase (U/l), AST= Aspartate aminotransferase (U/l), ALT= Alanine aminotransferase (U/l).
Cholesterol and globulin levels were significantly lower (p < 0.05) in fish fed with a PPE-supplemented diet compared to the control. Notably, all three PPE groups (0.5%, 1.0%, and 1.5%) presented significantly lower cholesterol levels than the control group (p < 0.05). On the other hand, PPE-supplemented groups displayed significantly increased globulin levels compared to the control group (p < 0.05). However, no significant differences were observed in TP and Alb levels. Liver enzyme activities (ALP, AST, and ALT) in the PPE-supplemented groups were significantly lower (p < 0.05) than in the control group.
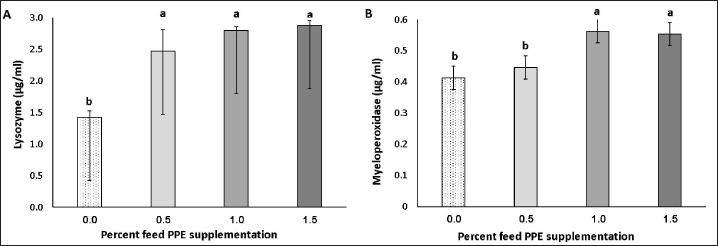
Lysozyme and MPO activities were higher in C. carpio fed on feed supplemented with PPE (Fig. 1). The lysozyme in PPE-supplemented groups increased with increasing PPE content (p < 0.05). Furthermore, MPO increased in the blood of common carp fed diets with 1.0% or 1.5% PPE (p < 0.05).
Fig. 1. (A) Lysozyme and (b) MPO levels of common carp, C. carpio, fed a diet supplemented with different concentrations of PPE for 60 days. Values with a different lowercase letter show a significant difference (p < 0.05); bar = standard deviation.
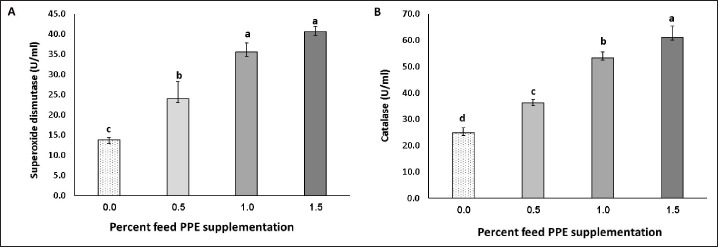
The antioxidant activity of SOD and CAT levels increased with increasing concentration of PPE in the diet. This increase was significant even in the 0.5% PPE group (Fig. 2).
Fig. 2. (A) Superoxide dismutase (SOD) and (B) catalase (CAT) levels in common carp, C. carpio, fed with PPE-supplemented feed at different concentrations for 70 days. Values with different lowercase letters show a significant difference (p < 0.05); error bars = standard deviation.
Discussion
Aeromonas veronii is a widely spread pathogen in aquaculture that can infect a variety of aquatic animals. It is a rod-shaped, mesophilic, facultative anaerobic bacterium (Su et al., 2023). This species has been linked to biliary sepsis and diarrhea in humans (Fernández-Bravo and Figueras, 2020). The biochemical traits of A. veronii isolated in our study are consistent with those identified by Hickman-Brenner et al. (1987), including the utilization of sugars such as maltose, mannose, glucose, and sucrose. Additionally, it showed positive reactions to lysine and ornithine decarboxylase and negative reactions to arginine dihydrolase. These results align with research on infected crucian carp (Chen et al., 2019) and Nile tilapia (El Latif et al., 2019), which reported similar morphological and biochemical characteristics of A. veronii in infected fish.
Plant waste compounds exhibit biological activities; therefore, this study evaluated the potential benefit of PPE as a feed additive to protect C. carpio against A. veronii. The results indicate significant growth improvement (FBW, WG, SGR, RGR, and PER) in common carp at 0.5% and 1% PPE feed concentration. However, the 1.5% PPE group showed decreased growth performance, similar to the control group, possibly due to the high levels of polyphenols and fiber (Ji et al., 2018).
FCR decreased with reduced PPE concentration, likely due to enhanced caloric intake and nutrient digestibility, while at 1% and 1.5% PPE, FCR was similar to the control group, possibly due to toxic components and excessive doses of polyphenols and fiber (Mahmoud et al., 2011; Fayed et al., 2012; Avazeh et al., 2020). Additionally, the antioxidant activities (SOD and CAT) were higher in fish fed on a PPE-supplemented feed, which enhances growth even in starvation conditions (Tejas et al., 2012). Studies have indicated that herbal medicines can act as appetite and growth stimulants by enhancing digestive enzymes, thereby increasing the growth and survival of fish (Srichaiyo et al., 2020; Reverter et al., 2021; Van Hai, 2015). Likewise, Abdel-Rahman et al. (2020) and Avazeh et al. (2020) observed growth improvement in Nile tilapia and rainbow trout when lower concentrations of PPE were used, as in our study. On the other hand, Badawi and Gomaa (2016) found no significant difference in SGR and FCR in Nile tilapia (O. niloticus) fed diets with varying PPE levels compared to the control group. High levels of PPE also affected positively PER in Nile tilapia (Toutou et al., 2019).
Hematological analyses are valuable tools in aquaculture and veterinary practice, providing insights into optimizing health. Hematological parameters are known to be highly sensitive to various environmental factors such as nutrition, water quality, stress, and pathogen exposure (Witeska et al., 2022). The current results demonstrate a significant increase in Hct and RBCs in common carp fed a PPE-supplemented diet compared to the control group. This increase likely indicates increases in RBC volume and hemoglobin concentration, potentially enhancing the fish’s oxygen-carrying capacity. Similar increases in Hct with PPE supplementation have been documented in olive flounder (Harikrishnan et al., 2012) and rainbow trout (Avazeh et al., 2020 and 2021). Moreover, the addition of PPE to common carp diets in our study increased RBC count, which agrees with findings by Acar et al. (2018), who reported increased RBCs and hemoglobin levels in rainbow trout fed diets enriched with pomegranate seed oil, suggesting that it improves oxygen transport and promotes better tissue perfusion.
Furthermore, WBCs play an integral role as components of innate and acquired immunity (Farrell, 2011; Esmaeili, 2021). We observed an increase in WBC at all PPE supplement concentrations. Harikrishnan et al. (2012) noted that olive flounder fed on pomegranate meal had resistance against diseases due to the increased WBC. However, Hrubec et al. (2000) demonstrated that exposure to a high bacterial load in low-quality water can negatively impact WBC count. Our results agree with Shafiei et al. (2016), Badrey et al. (2019), and Avazeh et al. (2020), who observed increased WBC counts in fish fed diets supplemented with PPE
TP in blood serum is a key indicator of fish health, reflecting their nutritional status and metabolism. It is influenced by factors such as species, age, sex, water temperature, and dietary quality and quantity (Dorojan et al., 2015). In our study, no significant differences were observed in TP and Alb levels among the groups, aligning with Shafiei et al. (2016) and Sayed-Lafi et al. (2022). However, Badrey et al. (2019) reported an increase in TP in Nile tilapia-fed PPE.
Globulins play a vital role in the immune system (Wiegertjes et al., 1996), and an increase in globulin levels suggests an enhanced immune response and improved resistance to infection (Løvoll et al., 2006). Consistently, the current results show a significant elevation in globulin levels in the dietary PPE groups, likely due to the immunostimulant properties of pomegranate (Dügenci et al., 2003).
The cholesterol levels decreased in the PPE groups compared to the control group, indicating good liver health and reduced lipid oxidation due to antioxidant activity (Forgione et al., 2024). This effect could vary with fish species and feeding duration (Avazeh et al., 2021). Likewise, Badrey et al. (2019) noted decreases in triglycerides and total cholesterol in Nile tilapia-fed pomegranate. However, Avazeh et al. (2021) observed that PPE increased cholesterol levels in rainbow trout.
Liver enzymes such as ALP, AST, and ALT are used as biomarkers to detect and monitor liver damage (Akbary et al., 2018). In our study, liver enzyme levels were elevated in the control group of common carp due to infection with A. veronii. In contrast, the groups treated with PPE displayed reduced liver enzyme levels compared to the control. PPE could have protected the hepato-cellular membrane and the normal histological structure of the liver by the antioxidant properties of phenols and flavonoids (Friedman, 2000; Cao et al., 2016). These findings suggest that PPE could combat bacterial infections and improve fish health by mitigating liver damage and decreasing liver enzyme levels. Similarly, Chattopadhyay (2003) reported that leaf extract of Indian lilac, Azadirachta indica, can help maintain liver health. The current findings concur with other studies (Badawi and Gmaa, 2016; Shafiei et al., 2016; Acar et al., 2018; Badrey et al., 2019).
This study demonstrates that incorporating PPE in fish feed strengthens the immune system by increasing SOD and CAT levels. Furthermore, it is known that pomegranate peel has antibacterial properties by virtue of active compounds that can destroy the cell membrane of bacteria (Hamady et al., 2015). Pomegranates contain antibacterial agents, such as pelargonidin-3-galactose, cyanidin-3-glucose, quercetin, and myricetin (Naz et al., 2007), as well as others such as flavonols, gallotannins and ellagic acid derivatives (Dahham et al., 2010). These compounds are effective against both Gram-negative and Gram-positive bacteria, such as Bacillus sp, Shigella sp, Salmonella sp, Staphylococcus sp, Vibrio cholera, Escherichia coli, Pseudomonas aeruginosa, and Aeromonas hydrophila (Hama et al., 2014; Tinrat and Singhapol, 2014; Hassan et al., 2018; Abdel-Rahman et al., 2020).
Furthermore, incorporating medicinal plants in feed stimulates the defense mechanisms of fish against some microbes (Giri et al., 2015; Hoseinifar et al., 2019). Harikrishnan et al. (2012) reported that a diet rich in pomegranates boosts the innate immunity of olive flounder against Philasterides dicentrarchi. The present results do not conflict with previous findings that pomegranate consumption can enhance the innate immunity of other fish species (Harikrishnan et al., 2012; Acar et al., 2018; Monir et al., 2020).
The antioxidant system is crucial for eliminating reactive oxygen species, preventing cell damage. In this context, lysozyme plays a vital role in innate immunity by breaking down the cell walls of bacteria, while MPO is involved in responding to microbial infections (Hoseinifar et al., 2019). Our results demonstrate increased lysozyme and MPO activity in fish fed on PPE-supplemented diets, indicating a positive impact on their immune responses. This might be attributed to the presence of phenolic compounds in PPE, such as protochatechuic acid, gallic acid, pyrogallol, p-coumaric acid, catechin, rosmarinic acid, rutin, naringenin, myricetin, scopoletin, and hesperidin, which possess antioxidant activities that reduce oxidative stress, free radical generation, and lipid peroxidation (Mashkor and Muhson, 2014; Azmat et al., 2024; Ranjana et al., 2024). PPE also contains ellagitannins, which are known to stimulate the growth of lymphocytes (Fraga, 2007). Consequently, the infected fish fed on PPE diets appeared to be in better health in our study. Our results are consistent with Harikrishnan et al., (2012); Shafiei et al., (2016), and Hamed and Abdel-Tawwab (2021).
Conclusion
Supplementing fish food with PPE benefits common carp by supporting their growth, boosting their immune system, and enhancing their antioxidant defenses against Aeromonas veronii. We propose that PPE could be a promising alternative to antibiotics and chemotherapeutics in fish farming. Further research is needed to fully understand the mechanisms of PPE’s impact and identify the optimal dosage for improving growth performance and health in common carp.
Acknowledgments
The authors thank the Department of Fish and Marine Resources, College of Agriculture, University of Basrah, Iraq, for logistical support to complete the present work.
Conflict of interest
The authors declare that there is no conflict of interest.
Funding
No funding was received for this study. The authors funded the study themselves.
Authors contributions
RMS-L planned the experimental design and executed the in vitro and in vivo experiments. FAS entered the raw data and carried out statistical analyses using SPSS. HHS shared in the writing, checked the tables, and constructed the figures. NAHA isolated and identified the bacteria. RMS-L and HHS conducted the literature review, drafted and revised the literature, and wrote the final manuscript. All authors read and approved the article.
Data availability
All the data are included in the manuscript.
References
- Abdel-Latif, H.M.R., Abdel-Tawwab, M., Khafaga, A.F. and Dawood, M.A.O. 2020. Dietary oregano essential oil improved the growth performance via enhancing the intestinal morphometry and hepato-renal functions of common carp (Cyprinus carpio L.) fingerlings. Aquac. 526, 735432. [Google Scholar]
- doi: 10.1186/s12864-017-3557-1. Abdelrahman, H. and ElHady, M. et al. 2017. Aquaculture genomics, genetics and breeding in the United States: current status, challenges, and priorities for future research. BMC Genom. 18, 191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- doi: 10.3389/fphys.2018.00596. Acar, Ü., Parrino, V., Kesbiç, O.S., Lo Paro, G., Saoca, C., Abbate, F., Yılmaz, S. and Fazio, F. 2018. Effects of different levels of pomegranate seed oil on some blood parameters and disease resistance against Yersinia ruckeri in rainbow trout. Front. Physiol. 9, 596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aebi, H. 1974. Catalase. In Methods of enzymatic analysis. Eds., Bergmeyer HU, Gawehn K. Cambridge, MA: Academic press, pp: 673–684. [Google Scholar]
- doi: 10.1002/fsn3.3320. Ain, H.B.U., Tufail, T., Bashir, S., Ijaz, N., Hussain, M., Ikram, A., Farooq, M.A. and Saewan, S.A. 2023. Nutritional importance and industrial uses of pomegranate peel: a critical review. Food Sci. Nutr. 11(6), 2589–2598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- doi: 10.1007/s11356-017-0582-1. Akbary, P., Sartipi Yarahmadi, S. and Jahanbakhshi, A. 2018. Hematological, hepatic enzymes’ activity and oxidative stress responses of gray mullet (Mugil cephalus) after sub-acute exposure to copper oxide. ESPR. 25(2), 1800–1808. . [DOI] [PubMed] [Google Scholar]
- doi: 10.1016/j.foodchem.2014.11.035. Akhtar, S., Ismail, T., Fraternale, D. and Sestili, P. 2015. Pomegranate peel and peel extracts: chemistry and food features. Food Chem. 174, 417–425. [DOI] [PubMed] [Google Scholar]
- Ali, M.F., Rashid, M.M., Rahman, M.M. and Haque, M.N. 2014. Pathogenicity of Aeromonas hydrophila in silver carp Hypophthalmichthys molitrix and its control trial. IOSR-JAVS. 7(6), 21–24. [Google Scholar]
- AOAC (2005) Association of Official Analytical Chemist, Official Methods of Analysis. 18th Edition, Gaithersburg, MD: AOAC. [Google Scholar]
- Avazeh, A., Adel, M., Shekarabi, S.P.H., Emamadi, H., Dawood, M.A., Omidi, A.H. and Bavarsad, M. 2021. Effects of dietary pomegranate peel meal on the growth performance, blood indices, and innate immune response of rainbow trout (Oncorhynchus mykiss). Ann. Anim. Sci. 21(1), 233–244. [Google Scholar]
- Avazeh, A., Emadi, H., Salehifarsani, A., Hosseini Shekarabi, S.P., Negarestan, H. and Bavarsad, M. 2020. Effects of the pomegranate powder on the body composition, hematological and biochemical indices of the rainbow trout (Oncorhynchus mykiss). JARD. 14(3),13–27. [Google Scholar]
- doi: 10.1002/fsn3.3777. Azmat, F., Safdar, M., Ahmad, H., Khan, M.R.J., Abid, J., Naseer, M.S., Aggarwal, S., Imran, A., Khalid, U., Zahra, S.M. and Islam, F., 2024. Phytochemical profile, nutritional composition of pomegranate peel and peel extract as a potential source of nutraceutical: a comprehensive review. Food Sci. 12(2), 661–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badawi, M.E. and Gomaa, A.M. 2016. Influence of diets supplemented with pomegranate peel extract on performance in Oreochromus niloticus. Japanese J. Vet. Res. 64(Suppl. 2), S87–S94. [Google Scholar]
- Badrey, A.A., Osman, A., Farrag, S.M., Toutou, M.M.M. and Moustafa, M. 2019. Influences of diets supplemented with pomegranate peel on haematology, blood biochemistry and immune status in monosex Nile tilapia, Oreochromis niloticus. EJABF. 23(2), 133–144. [Google Scholar]
- doi: 10.3389/fendo.2019.00062. Balasch, J.C. and Tort, L. 2019. Netting the stress responses in fish. Front. Endocrinol. 10, 435714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- doi: 10.3390/pathogens12040598. Behera, B.K., Parida, S.N., Kumar, V., Swain, H.S., Parida, P.K., Bisai, K., Dhar, S. and Das, B.K. 2023. Aeromonas veronii is a lethal pathogen isolated from gut of infected Labeo rohita: molecular insight to understand the bacterial virulence and its induced host immunity. Pathog. 12(4), 598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergmeyer, H.U., Gawehn, K. and Grassl, M. 1976. Enzyme profiles of liver and serum in crammed geese. Clin. Chem. 22(4), 552–558. [Google Scholar]
- Boyd, C.E. and Massaut, L. 1999. Risks associated with the use of chemicals in pond aquaculture. Aquac. Eng. 20(2), 113–132. [Google Scholar]
- Braun, N., de Lima, R.L., Baldisserotto, B., Dafre, A.L. and de Oliveira Nuñer, A.P. 2010. Growth, biochemical and physiological responses of Salminus brasiliensis with different stocking densities and handling. Aquac. 301(1-4), 22–30. [Google Scholar]
- doi: 10.1002/fsn3.4207. Caglak, E., Ogretmen, O.Y. and Karsli, B. 2024. The effect of pomegranate peel extract added as a natural preservative on the quality parameters of thornback ray (Raja clavata) sausages stored at +4°C. Food Sci. Nutr. 12(8), 6011–6021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao, L., Du, J., Ding, W., Jia, R., Liu, Y., Xu, P., Teraoka, H. and Yin, G. 2016. Hepatoprotective and antioxidant effects of dietary Angelica sinensis extract against carbon tetrachloride-induced hepatic injury in Jian Carp (Cyprinus carpio var. Jian). Aquac. Res. 47(6), 1852–1863. [Google Scholar]
- Chang, S., Wang, J., Dong, C. and Jiang, Y. 2023. Intestinal microbiota signatures of common carp (Cyprinus carpio) after the infection of Aeromonas hydrophila. Aquac. Rep. 30, 101585. [Google Scholar]
- doi: 10.1016/j.jep.2003.08.006. Chattopadhyay, R. 2003. Possible mechanism of hepatoprotective activity of Azadirachta indica leaf extract, part II. J. Ethnopharmacol. 89(2-3), 217–219. [DOI] [PubMed] [Google Scholar]
- Chen, C.Z., Li, P., Wang, W.B. and Li, Z.H. 2022. Response of growth performance, serum biochemical parameters, antioxidant capacity, and digestive enzyme activity to different feeding strategies in common carp (Cyprinus carpio) under high-temperature stress. Aquac. 548, 737636. [Google Scholar]
- doi: 10.3389/fmicb.2019.02742. Chen, F., Sun, J., Han, Z., Yang, X., Xian, J.A., Lv, A., Hu, X. and Shi, H. 2019. Isolation, identification and characteristics of Aeromonas veronii from diseased crucian carp (Carassius auratus gibelio). Front. microbiol. 10, 2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahham, S.S., Ali, M.N., Tabassum, H. and Khan, M. 2010. Studies on antibacterial and antifungal activity of pomegranate (Punica granatum L.). Am. Eurasian J. Agric. Environ. Sci. 9(3), 273–281. . [Google Scholar]
- Dawood, M.A.O., Koshio, S. and Esteban, M.Á. 2018. Beneficial roles of feed additives as immunostimulants in aquaculture: a review. Rev. Aquac. 10, 950–974. [Google Scholar]
- Decie, S.I.V. and Lewis, S.M. 2006. Practical hematology. 10th ed. London, UK: Churchill Livingstone, 13, pp. 978–443. [Google Scholar]
- Dorojan, O.G.V., Cristea, V., Creţu, M., Coadă, M.T., Dediu, L., Grecu, I.R. and Plăcintă, S. 2015. Effect of thyme (Thymus vulgaris) and vitamin E on growth performance and body composition of Acipenser stellatus juveniles. Aquacult. Aquarium Conserv. Legis. 8(2), 195–202. [Google Scholar]
- doi: 10.1016/s0378-8741(03)00182-x. Dügenci, S.K., Arda, N. and Candan, A. 2003. Some medicinal plants as immunostimulant for fish. J. Ethnopharmacol. 88(1), 99–106. [DOI] [PubMed] [Google Scholar]
- El Latif, A.A., Elabd, H., Amin, A., Eldeen, A.N. and Shaheen, A.A. 2019. High mortalities caused by Aeromonas veronii: identification, pathogenicity, and histopathological studies in Oreochromis niloticus. Aquac Int. 27, 1725–1737. [Google Scholar]
- doi: 10.3390/biology10121236. Esmaeili, M. 2021. Blood performance, a new formula for fish growth and health. Biol. 10(12), 1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FAO. 2020. The State of World Fisheries and Aquaculture. Sustainability in action. Rome, Italy: FAO; doi: 10.4060/ca9229en. [Google Scholar]
- FAO. 2022. The State of World Fisheries and Aquaculture 2022: towards blue transformation Food and Agriculture Organization of the United Nations, Rome, Italy: FAO. [Google Scholar]
- Farrell, A.P. 2011. Encyclopedia of fish physiology from genome to environment. 1st edition. Cambridge, MA: Academic Press, pp. 984–991. [Google Scholar]
- Fayed A.M., Azoz, A.A., Zedan, A.H. and Basyony, M. 2012. Effects of pomegranate peel as antioxidant supplementation on digestibility, blood biochemical and rabbit semen quality. Egypt J. Nutr. Feeds 15, 343–354. [Google Scholar]
- doi: 10.3390/microorganisms8010129. Fernández-Bravo, A. and Figueras, M.J. 2020. An update on the genus Aeromonas: taxonomy, epidemiology, and pathogenicity. Microorganisms 8(1), 129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- doi: 10.3390/microorganisms12071303. Forgione, G., De Cristofaro, G.A., Sateriale, D., Pagliuca, C., Colicchio, R., Salvatore, P., Paolucci, M. and Pagliarulo, C. 2024. Pomegranate peel and olive leaf extracts to optimize the preservation of fresh meat: natural food additives to extend shelf-life. Microorganisms 12(7), 1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- doi: 10.1080/15216540701230529. Fraga, C.G. 2007. Plant polyphenols, how to translate their in vitro antioxidant actions to in vivo conditions. IUBMB Life 59(4-5), 308–315. [DOI] [PubMed] [Google Scholar]
- doi: 10.1074/jbc.275.4.2247. Friedman, S.L. 2000. Molecular regulation of hepatic fibrosis, an integrated cellular response to tissue injury. J. Biol. Chem. 275(4), 2247–2250. [DOI] [PubMed] [Google Scholar]
- Ghasemi, R., Akrami Mohajeri, F., Heydari, A., Yasini, S.A., Dehghani Tafti, A. and Khalili Sadrabad, E. 2023. Application of pomegranate peel extract, a waste agricultural product, as a natural preservative in tahini. Int. J. Food Sci. (1), 8860476. [Google Scholar]
- doi: 10.1016/j.fsi.2015.05.051. Giri, S.S., Sen, S.S., Chi, C., Kim, H.J., Yun, S., Park, S.C. and Sukumaran, V. 2015. Effect of guava leaves on the growth performance and cytokine gene expression of Labeo rohita and its susceptibility to Aeromonas hydrophila infection. Fish Shellfish Immunol. 46(2), 217–224. [DOI] [PubMed] [Google Scholar]
- Gupta, S.K., Gupta, A., Sarkar, B., Gupta, R., Kumar, M., Kumari, A. and Foysal, M.J. 2023. Pomegranate (Punica granatum) peel extract supplementation in diet influences growth performance, haemato-immunological responses and cytokine expression in pathogen-aggravated Labeo rohita fingerlings. Aquac. 562, 738823. [Google Scholar]
- Hama, A.A., Taha, Y. and Qadir, S.A. 2014. The antimicrobial activity of pomegranate (Punica granatum) juice. IJSER. 5(10), 796–798. [Google Scholar]
- Hamady, G.A., Abdel-Moneim, M.A., El-Chaghaby, G.A., Abd-El-Ghany, Z.M. and Hassanin, M.S. 2015. Effect of pomegranate peel extract as natural growth promoter on the productive performance and intestinal microbiota of broiler chickens. AJAST. 3(12), 514–519. [Google Scholar]
- doi: 10.1016/j.cbpc.2021.109067. Hamed, H.S. and Abdel-Tawwab, M. 2021. Dietary pomegranate (Punica granatum) peel mitigated the adverse effects of silver nanoparticles on the performance, haemato-biochemical, antioxidant, and immune responses of Nile tilapia fingerlings. Aquac. 540, 736742. [DOI] [PubMed] [Google Scholar]
- Harikrishnan, R., Balasundaram, C. and Heo, M. S. 2011. Impact of plant products on innate and adaptive immune system of cultured finfish and shellfish. Aquac. 317(1-4), 1–15. [Google Scholar]
- doi: 10.1016/j.vetpar.2011.12.006. Harikrishnan, R., Kim, J.S., Kim, M.C., Balasundaram, C. and Heo, M.S. 2012. Pomegranate enriched diet enhances the hematology, innate immune response, and disease resistance in olive flounder against Philasterides dicentrarchi. Vet. Parasitol. 187(1-2), 147–156. [DOI] [PubMed] [Google Scholar]
- Hassan, S.M., Hamad, A.K. and Shallal, A.F. 2018. The effect of pomegranate extracts on bacteria. J. Raparin Univ. 5(15), 5–18. [Google Scholar]
- doi: 10.1128/jcm.25.5.900-906.1987. Hickman-Brenner, F.W., MacDonald, K.L., Steigerwalt, A.G., Fanning, G.R., Brenner, D.J. and Farmer 3rd, J.J. 1987. Aeromonas veronii, a new ornithine decarboxylase-positive species that may cause diarrhea. J. Clin. Microbiol. 25(5), 900–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- doi: 10.1016/j.fsi.2018.12.001. Hoseinifar, S.H., Sohrabi, A., Paknejad, H., Jafari, V., Paolucci, M. and Van Doan, H. 2019. Enrichment of common carp (Cyprinus carpio) fingerlings diet with Psidium guajava: the effects on cutaneous mucosal and serum immune parameters and immune related genes expression. Fish Shellfish Immunol. 86, 688–694. [DOI] [PubMed] [Google Scholar]
- doi: 10.1111/j.1939-165x.2000.tb00389.x. Hrubec, T.C., Cardinale, J.L. and Smith, S.A. 2000. Hematology and plasma chemistry reference intervals for cultured tilapia (Oreochromis hybrid). Vet. Clin. Pathol. 29(1), 7–12. [DOI] [PubMed] [Google Scholar]
- doi: 10.1016/j.jep.2012.07.004. Ismail, T., Sestili, P. and Akhtar, S. 2012. Pomegranate peel and fruit extracts: a review of potential anti-inflammatory and anti-infective effects. J. Ethnopharmacol. 143(2), 397–405. [DOI] [PubMed] [Google Scholar]
- Ji, R., Li, Y., Li, X., Xiang, X., Li, Y., Zhu, S., Yang B., Zhang Y., Mai, K. and Ai, Q. 2018. Effects of dietary tea polyphenols on growth, biochemical and antioxidant responses, fatty acid composition and expression of lipid metabolism related genes of large yellow croaker (Larimichthys crocea). Aquac. Res. 49(3), 1210–1218. [Google Scholar]
- doi: 10.1007/s00251-006-0096-3. Løvoll, M., Kilvik, T., Boshra, H., Bøgwald, J., Sunyer, J.O. and Dalmo, R.A. 2006. Maternal transfer of complement components C3-1, C3-3, C3-4, C4, C5, C7, Bf, and Df to offspring in rainbow trout (Oncorhynchus mykiss). Immunogenet. 58, 168–179. [DOI] [PubMed] [Google Scholar]
- Mahmoud, M.H., Kassem, S.S., Abdel-Kader, M.M. and El-Shobaki, F.A. 2011. How to reduce weight and keep healthy. t. J. Acad. Res. 3(6), 126–132. [Google Scholar]
- Mashkor, I.M.A.A. and Muhson, A.A. 2014. Total phenol, total flavonoids and antioxidant activity of pomegranate peel. Int. J. Chem.Tech. Res. 6(11), 4656–4661. [Google Scholar]
- Medina-Morillo, M., Sotil, G., Arteaga, C., Cordero, G., Martins, M.L., Murrieta-Morey, G. and Yunis-Aguinaga, J. 2023. Pathogenic Aeromonas spp in Amazonian fish: virulence genes and susceptibility in Piaractus brachypomus, the main native aquaculture species in Peru. Aquac. Rep. 33, 101811. [Google Scholar]
- doi: 10.1016/j.micpath.2020.104202. Monir, W., Abdel-Rahman, M.A., Hassan, S.E.D. and Awad, S.M. 2020. Pomegranate peel and moringa-based diets enhanced biochemical and immune parameters of Nile tilapia against bacterial infection by Aeromonas hydrophila. Microb. Pathog. 145, 104202. [DOI] [PubMed] [Google Scholar]
- doi: 10.1111/tbed.13482. Muniesa, A., Basurco, B., Aguilera, C., Furones, D., Reverté, C., Sanjuan-Vilaplana, A., Jansen, M.D., Brun, E. and Tavornpanich, S. 2020. Mapping the knowledge of the main diseases affecting sea bass and sea bream in Mediterranean. Transbound. Emerg. Dis. 67(3), 1089–1100. [DOI] [PubMed] [Google Scholar]
- Natt, M.P. and Herrick, C.A. 1952. A new blood diluent for counting the erythrocytes and leucocytes of the chicken. Poult. Sci. 31(4), 735–738. [Google Scholar]
- doi: 10.1111/j.1750-3841.2007.00533.x. Naz, S., Siddiqi, R., Ahmad, S., Rasool, S.A. and Sayeed, S.A. 2007. Antibacterial activity directed isolation of compounds from Punica granatum. J. Food Sci. 72(9), M341–M345. [DOI] [PubMed] [Google Scholar]
- doi: 10.1007/s12328-017-0813-5. Nishida, A.. Inoue, R., Inatomi, O., Bamba, S., Naito, Y. and Andoh, A. 2018. Gut microbiota in the pathogenesis of inflammatory bowel disease. Clin. J. Gastroenterol. 11, 1–10. [DOI] [PubMed] [Google Scholar]
- doi: 10.1016/s0006-291x(72)80218-3. Nishikimi, M., Rao, N.A. and Yagi, K. 1972. The occurrence of superoxide anion in the reaction of reduced phenazine methosulfate and molecular oxygen. Biochem. Biophys. Res. Commun. 46(2), 849–854. [DOI] [PubMed] [Google Scholar]
- NRC (National Research Council). 1993. Nutrient Requirements of Fish. Washington, DC: National Academies Press, pp. 112. [Google Scholar]
- doi: 10.1016/j.jep.2010.10.044. Nudo, L.P. and Catap, E.S. 2011. Immunostimulatory effects of Uncaria perrottetii (A. Rich.) Merr. (Rubiaceae) vinebark aqueous extract in Balb/C mice. J. Ethnopharmacol. 133(2), 613–620. [DOI] [PubMed] [Google Scholar]
- Pantha, M.B. 1982. Integrated agro-fisheries activities in Nepal. In ICLARM Conference Proceedings (Philippines) (No. 8). [Google Scholar]
- doi: 10.3390/plants13020281. Radan, M., Ćujić Nikolić, N., Kuzmanović Nedeljković, S., Mutavski, Z., Krgović, N., Stević, T., Marković, S., Jovanović, A., Živković, J. and Šavikin, K. 2024. Multifunctional pomegranate peel microparticles with health-promoting effects for the sustainable development of novel nutraceuticals and pharmaceuticals. Plants, 13(2), 281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranjana, N., Haripriya, S. and Sundarapandian, M. 2024. Pomegranate powerhouse: a synthesis of scientific insights into its nutraceutical marvels and biomedical applications. IJSRST. 11(1), 456–469. [Google Scholar]
- RedCorn, R., Fatemi, S. and Engelberth, A.S. 2018. Comparing end-use potential for industrial food-waste sources. Engineering 4, 371–380. [Google Scholar]
- Reverter, M., Tapissier-Bontemps, N., Sarter, S., Sasal, P. and Caruso, D. 2021. Moving towards more sustainable aquaculture practices: a meta-analysis on the potential of plant-enriched diets to improve fish growth, immunity and disease resistance. Rev. Aquac. 13(1), 537–555. [Google Scholar]
- Sahoo, P.K., Pattanayak, S., Paul, A., Sahoo, M.K. and Pasim, R.K. 2020. Carp edema virus in ornamental fish farming in India: a potential threat to koi carps but not to co-cultured Indian major carp or goldfish. JEB. 58(4), 254–262. [Google Scholar]
- Sayed-Lafi, R.M., Al-Tameemi, R.A. and Sultan, F.A. 2022. Utilization tow extracts of pomegranate (Punica granatum) peel on growth performance and serum biochemical parameters of the common carp (Cyprinus carpio) fingerlings. EJABF. 26(6), 319–328. [Google Scholar]
- Shafiei, F., Soofiani, N.M., Ebrahim, E., Nematollahi, A. and Mohebbi, A. 2016. Effect of alcoholic extract of pomegranate peel (Punica granatum L.) on blood parameters of common carp (Cyprinus carpio) fingerling. Sci. Res. J. 5(2), 59–72. [Google Scholar]
- Song, X., Zhao, J., Bo, Y., Liu, Z., Wu, K. and Gong, C. 2014. Aeromonas hydrophila induces intestinal inflammation in grass carp (Ctenopharyngodon idella): an experimental model. Aquac. 434, 171–178. [Google Scholar]
- Srichaiyo, N., Tongsiri, S., Hoseinifar, S.H., Dawood, M.A., Jaturasitha, S., Esteban, M.Á., Ringø, E. and Van Doan, H. 2020. The effects gotu kola (Centella asiatica) powder on growth performance, skin mucus, and serum immunity of Nile tilapia (Oreochromis niloticus) fingerlings. Aquac. Res. 16, 100239. [Google Scholar]
- doi: 10.3390/ani13172779. Su, X., Yang, M., Li, Y., Yan, X., Hou, R., Ayala, J.E., Li, L., Yue, C., Zhang, D. and Liu, S. 2023. First Isolation and Identification of Aeromonas veronii in a captive giant panda (Ailuropoda melanoleuca). Animals, 13(17), 2779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tejas, G.H., Umang, J.H., Payal, B.N., Tusharbinu, D.R. and Pravin, T.R. 2012. A panoramic view on pharmacognostic, pharmacological, nutritional, therapeutic and prophylactic values of Moringa olifera Lam. Int. Res. J. Pharm. 3, 1–7. [Google Scholar]
- Tietz, N.W. 1990. Blood gases and electrolytes. In Fundamentals of Clinical Chemistry. Eds., Burtis CA, Bruns DE. Philadelphia, PA: Sounders, pp: 903–908. [Google Scholar]
- Tinrat, S. and Singhapol, C. 2014. Evaluation of antioxidant and antimicrobial activities of pomegranate (Punica granatum Linn.) peel extracts. KKU Res. J. 19(3), 353–360. [Google Scholar]
- Toutou, M.M., Farrag, M.M., Badrey, A.E. and Moustafa, M.A. 2019. Growth performance, feed utilization and gut histology of monosex Nile tilapia (Oreochromis niloticus) fed with varying levels of pomegranate (Punica granatum) peel residues. Aquacult. Aquarium Conserv. Legis. 12(1), 298–309. [Google Scholar]
- Urbinati, E.C., Zanuzzo, F.S. and Biller, J.D. 2020. Stress and immune system in fish. In Biology and physiology of freshwater neotropical fish. Eds., Baldisserotto, B., Urbinati, E.C. and Cyrino, J.E.P.: Academic Press, London, UK, pp: 93–114. [Google Scholar]
- Van Hai, N. 2015. The use of medicinal plants as immunostimulants in aquaculture, a review. Aquac. 446, 88–96. [Google Scholar]
- doi: 10.4103/2231-4040.154542. Vora, A.K., Londhe, V.Y. and Pandita N.S. 2015. Preparation and characterization of standardized pomegranate extract-phospholipid complex as an effective drug delivery tool. J. Adv. Pharm. Technol. Res. 6(2), 75–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- doi: 10.1016/s0145-305x(96)00032-8. Wiegertjes, G.F., Stet, R.M., Parmentier, H.K. and van Muiswinkel, W.B. 1996. Immunogenetics of disease resistance in fish: a comparative approach. Dev. Comp. Immunol. 20(6), 365–381. [DOI] [PubMed] [Google Scholar]
- Witeska, M., Kondera, E., Ługowska, K. and Bojarski, B. 2022. Hematological methods in fish–not only for beginners. Aquac. 547, 737498. [Google Scholar]
- Wotton, I.D. and Freeman, H. 1982. Microanalysis in medicinal biochemical. London, UK: Churchill Livingstone, pp: 1974. [Google Scholar]
- doi: 10.1016/j.ijfoodmicro.2009.07.002. Zoreky, N.S. 2009. Antimicrobial activity of pomegranate (Punica granatum L.) fruit peels. Int. J. Food Microbiol. 134, 244–248. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All the data are included in the manuscript.


