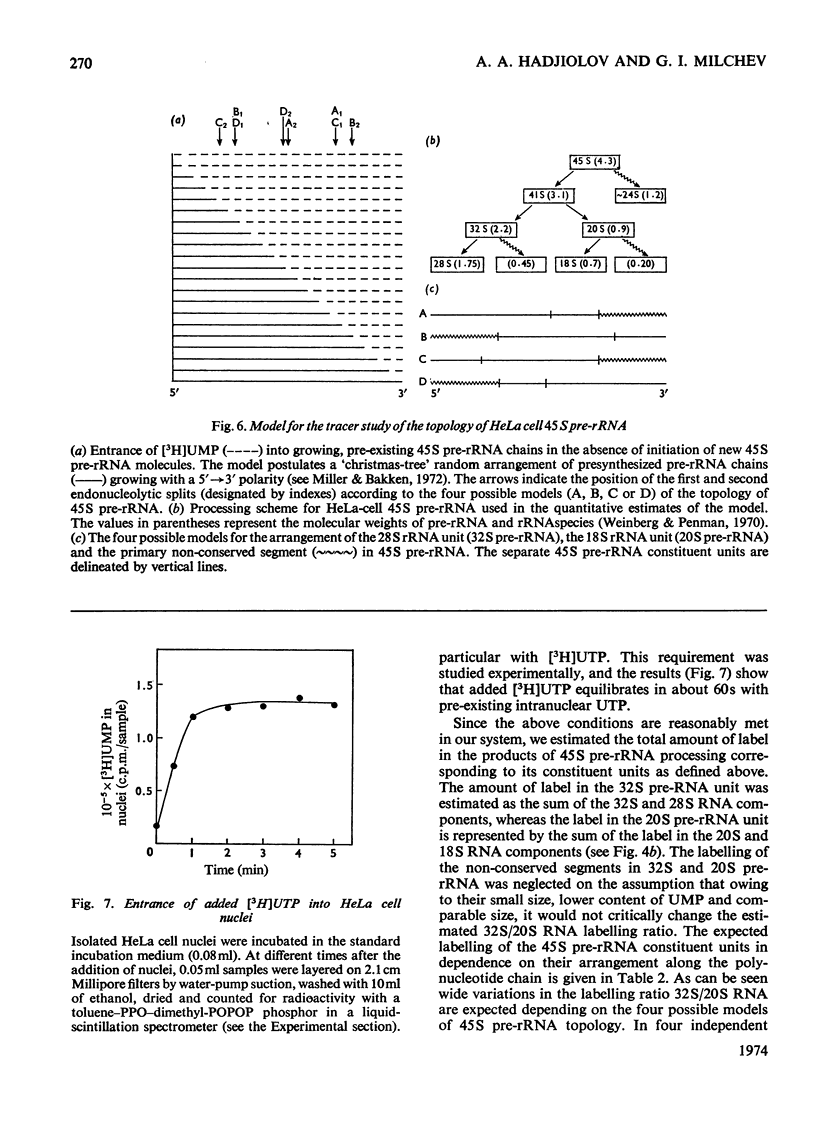
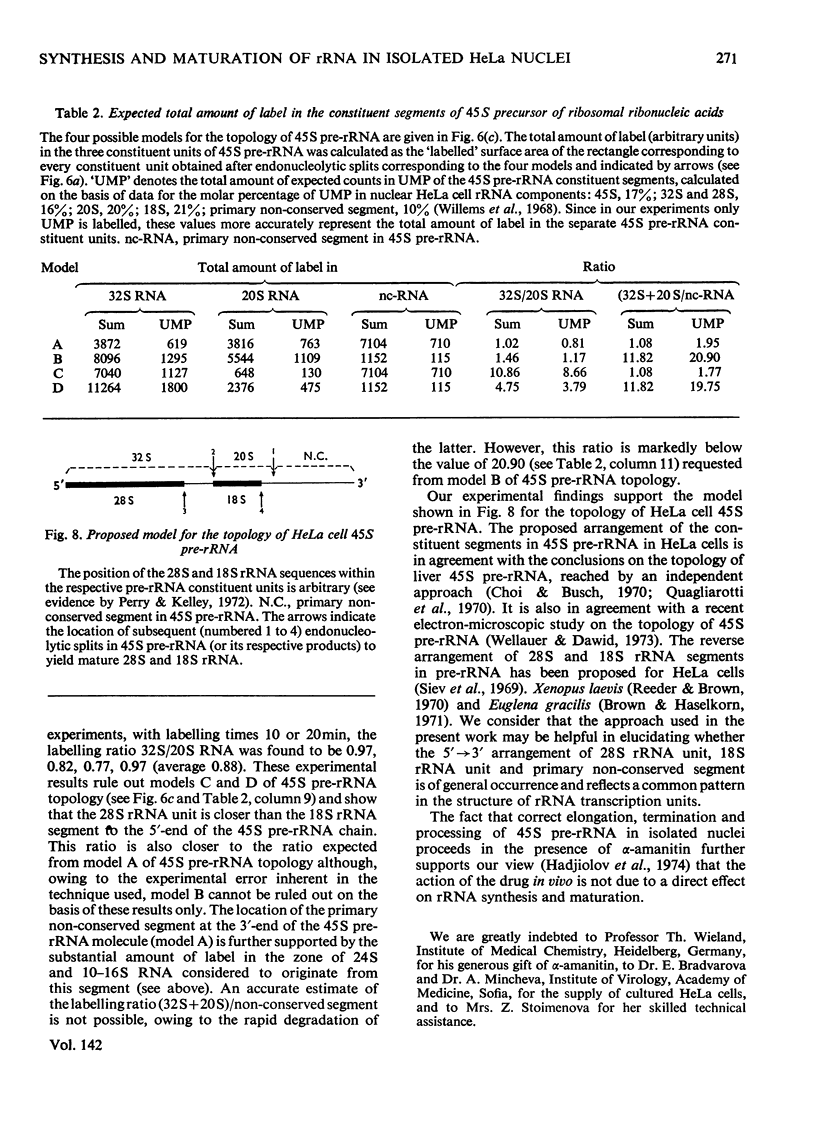
Abstract
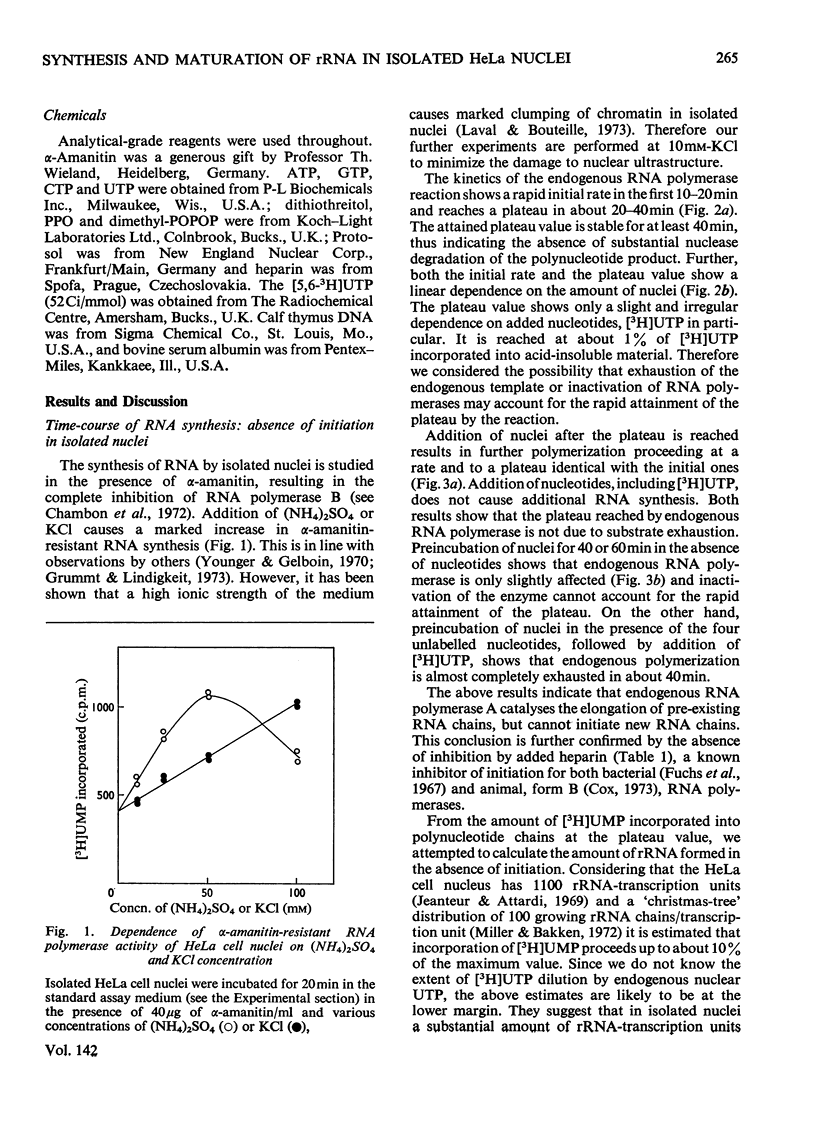
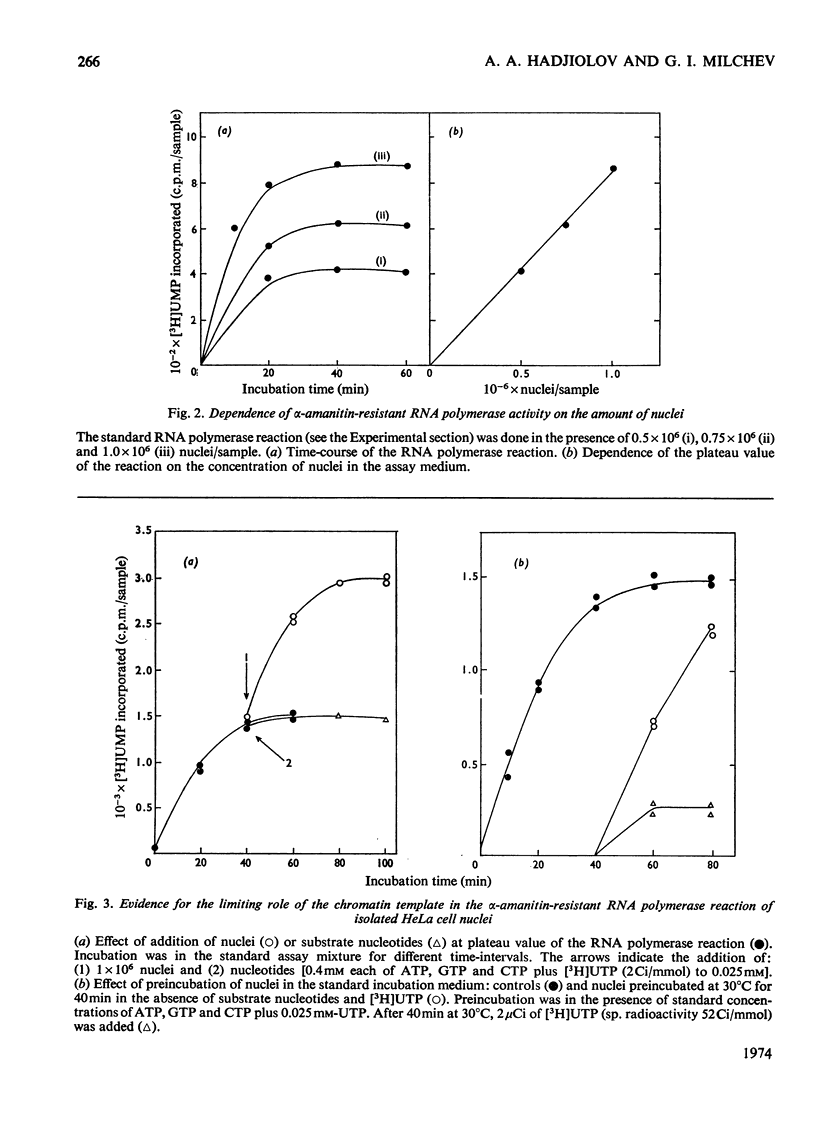
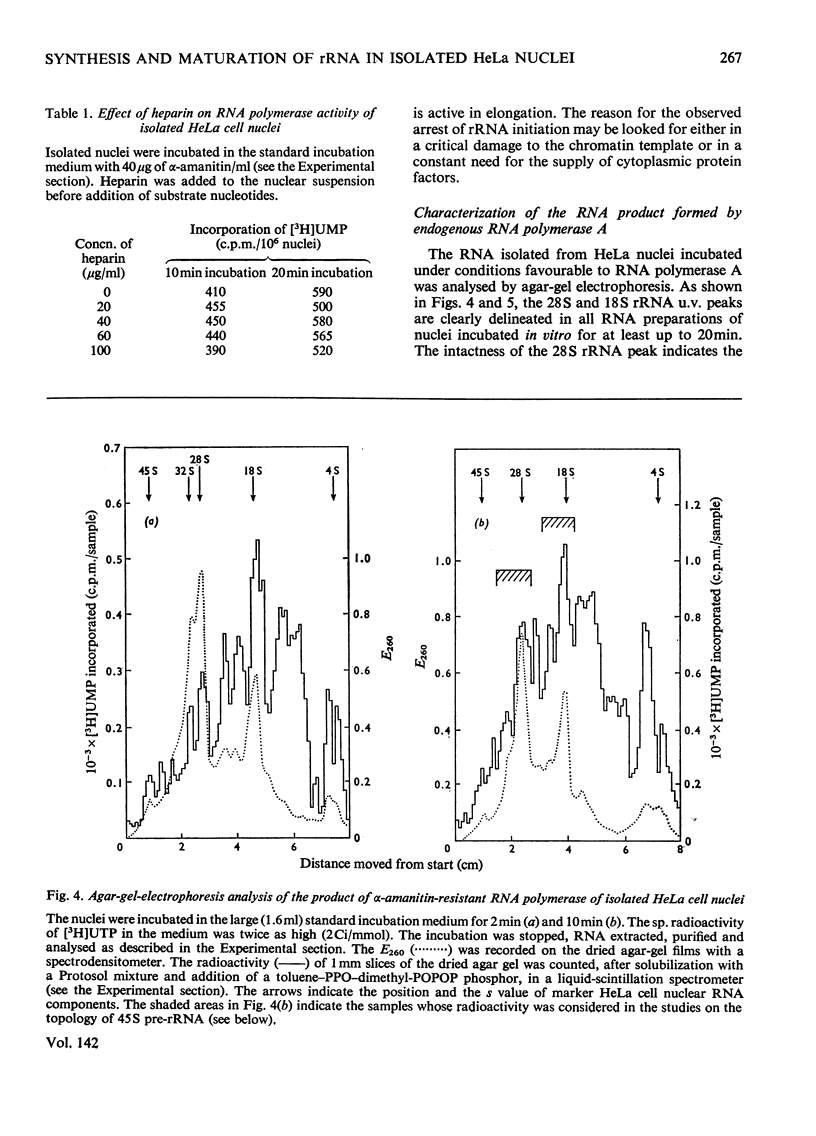
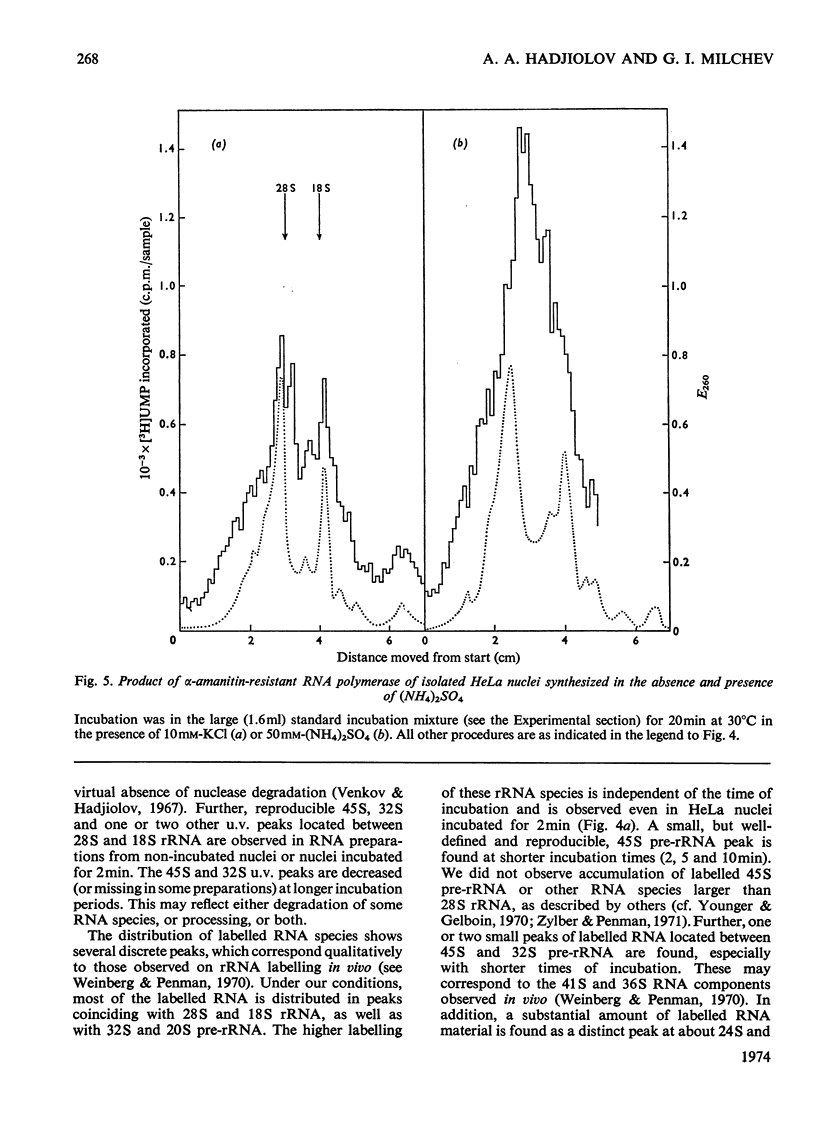
The synthesis and processing of RNA by isolated HeLa cell nuclei was studied at low ionic strength in the presence of α-amanitin. The RNA polymerase reaction, with endogenous template and enzyme, rapidly reaches a plateau dependent on the amount of nuclei. Evidence is presented that incorporation of [3H]UMP proceeds only in growing RNA chains, whereas initiation of new RNA chains is arrested. The product formed contains all the main components of the 45S pre-rRNA (precursor of rRNA) maturation pathway (45S, 32S and 20S pre-rRNA; 28S and 18S rRNA). Most of the labelled material is in the mature rRNA components and their immediate precursors, even at very short times of incubation (2min). Small, but definite, 5S and 4S RNA peaks are also observed. At shorter incubation times a substantial amount of [3H]UMP is incorporated into RNA molecules in the 24S and 10–16S zones. This RNA material is considered to represent the non-conserved segments of 45S pre-rRNA in the process of nucleolytic degradation. A model for the tracer study of the topology of 45S pre-rRNA, on arrest of rRNA initiation, is discussed. The experimental evidence obtained supports the following structure of 45S pre-rRNA: 5′-end–28S rRNA unit–18S rRNA unit–nonconserved segment–3′-end.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown R. D., Haselkorn R. Synthesis and maturation of cytoplasmic ribosomal RNA in Euglena gracilis. J Mol Biol. 1971 Aug 14;59(3):491–503. doi: 10.1016/0022-2836(71)90312-3. [DOI] [PubMed] [Google Scholar]
- Butterworth P. H., Cox R. F., Chesterton C. J. Transcription of mammalian chromatin by mammalian DNA-dependent RNA polymerases. Eur J Biochem. 1971 Nov 11;23(2):229–241. doi: 10.1111/j.1432-1033.1971.tb01613.x. [DOI] [PubMed] [Google Scholar]
- Caston J. D., Jones P. H. Synthesis and processing of high molecular weight RNA by nuclei isolated from embryos of Rana pipiens. J Mol Biol. 1972 Aug 14;69(1):19–38. doi: 10.1016/0022-2836(72)90021-6. [DOI] [PubMed] [Google Scholar]
- Chambon P., Gissinger F., Kedinger C., Mandel J. L., Meilhac M., Nuret P. Structural and functional properties of three mammalian nuclear DNA-dependent RNA polymerases. Acta Endocrinol Suppl (Copenh) 1972;168:222–246. doi: 10.1530/acta.0.071s222. [DOI] [PubMed] [Google Scholar]
- Choi Y. C., Busch H. Structural analysis of nucleolar precursors of ribosomal ribonucleic acid. Studies on the 5'-terminal and alkali-resistant dinucleotides of nucleolar high molecular weight ribonucleic acid. J Biol Chem. 1970 Apr 25;245(8):1954–1961. [PubMed] [Google Scholar]
- Cox R. F. Transcription of high-molecular-weight RNA from hen-oviduct chromatin by bacterial and endogenous form-B RNA polymerases. Eur J Biochem. 1973 Nov 1;39(1):49–61. doi: 10.1111/j.1432-1033.1973.tb03102.x. [DOI] [PubMed] [Google Scholar]
- Fuchse, Millette R. L., Zillig W., Walter G. Influence of salts on RNA synthesis by DNA-dependent RNA-polymerase from Escherichia coli. Eur J Biochem. 1967 Dec;3(2):183–193. doi: 10.1111/j.1432-1033.1967.tb19514.x. [DOI] [PubMed] [Google Scholar]
- Grummt I., Lindigkeit R. Pre-ribosomal RNA synthesis in isolated rat-liver nucleoli. Eur J Biochem. 1973 Jul 2;36(1):244–249. doi: 10.1111/j.1432-1033.1973.tb02906.x. [DOI] [PubMed] [Google Scholar]
- Hadjiolov A. A., Dabeva M. D., Mackedonski V. V. The action of alpha-amanitin in vivo on the synthesis and maturation of mouse liver ribonucleic acids. Biochem J. 1974 Mar;138(3):321–334. doi: 10.1042/bj1380321a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadjiolov A. A., Venkov P. V., Tsanev R. G. Ribonucleic acids fractionation by density-gradient centrifugation and by agar gel electrophoresis: a comparison. Anal Biochem. 1966 Nov;17(2):263–267. doi: 10.1016/0003-2697(66)90204-1. [DOI] [PubMed] [Google Scholar]
- Hecht R. M., Birnstiel M. L. Integrity of the DNA template, a prerequisite for the faithful transcription of Xenopus rDNA in vitro. Eur J Biochem. 1972 Sep 25;29(3):489–499. doi: 10.1111/j.1432-1033.1972.tb02013.x. [DOI] [PubMed] [Google Scholar]
- Jeanteur P., Attardi G. Relationship between HeLa cell ribosomal RNA and its precursors studied by high resolution RNA-DNA hybridization. J Mol Biol. 1969 Oct 28;45(2):305–324. doi: 10.1016/0022-2836(69)90107-7. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laval M., Bouteille M. Synthetic activity of isolated rat liver nuclei. I. Ultrastructural study at various steps of isolation. Exp Cell Res. 1973 Feb;76(2):337–348. doi: 10.1016/0014-4827(73)90385-6. [DOI] [PubMed] [Google Scholar]
- Maden B. E., Salim M., Summers D. F. Maturation pathway for ribosomal RNA in the Hela cell nucleolus. Nat New Biol. 1972 May 3;237(70):5–9. doi: 10.1038/newbio237005a0. [DOI] [PubMed] [Google Scholar]
- Marzluff W. F., Jr, Murphy E. C., Jr, Huang R. C. Transcription of ribonucleic acid in isolated mouse myeloma nuclei. Biochemistry. 1973 Aug 28;12(18):3440–3446. doi: 10.1021/bi00742a013. [DOI] [PubMed] [Google Scholar]
- Meilhac M., Chambon P. Animal DNA-dependent RNA polymerases. Initiation sites on calf-thymus DNA. Eur J Biochem. 1973 Jun 15;35(3):454–463. doi: 10.1111/j.1432-1033.1973.tb02859.x. [DOI] [PubMed] [Google Scholar]
- Miller O. L., Jr, Bakken A. H. Morphological studies of transcription. Acta Endocrinol Suppl (Copenh) 1972;168:155–177. doi: 10.1530/acta.0.071s155. [DOI] [PubMed] [Google Scholar]
- Perry R. P., Kelley D. E. The production of ribosomal RNA from high molecular weight precursors. 3. Hydrolysis of pre-ribosomal and ribosomal RNA by a 3'-OH specific exoribonuclease. J Mol Biol. 1972 Sep 28;70(2):265–279. doi: 10.1016/0022-2836(72)90538-4. [DOI] [PubMed] [Google Scholar]
- Pogo A. O., Littau V. C., Allfrey V. G., Mirsky A. E. Modification of ribonucleic Acid synthesis in nuclei isolated from normal and regenerating liver: some effects of salt and specific divalent cations. Proc Natl Acad Sci U S A. 1967 Mar;57(3):743–750. doi: 10.1073/pnas.57.3.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price R., Penman S. A distinct RNA polymerase activity, synthesizing 5-5 s, 5 s and 4 s RNA in nuclei from adenovirus 2-infected HeLa cells. J Mol Biol. 1972 Oct 14;70(3):435–450. doi: 10.1016/0022-2836(72)90551-7. [DOI] [PubMed] [Google Scholar]
- Reeder R. H., Roeder R. G. Ribosomal RNA synthesis in isolated nuclei. J Mol Biol. 1972 Jun 28;67(3):433–441. doi: 10.1016/0022-2836(72)90461-5. [DOI] [PubMed] [Google Scholar]
- Ro-Choi T. S., Moriyama Y., Choi Y. C., Busch H. Isolation and purification of a nuclear 4.4 S ribonucleic acid of the Novikoff hepatoma. J Biol Chem. 1970 Apr 25;245(8):1970–1977. [PubMed] [Google Scholar]
- Siev M., Weinberg R., Penman S. The selective interruption of nucleolar RNA synthesis in HeLa cells by cordycepin. J Cell Biol. 1969 May;41(2):510–520. doi: 10.1083/jcb.41.2.510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith K. E., Arnstein H. R. RNA synthesis in isolated rabbit-liver nuclei. Eur J Biochem. 1972 Oct 17;30(1):195–204. doi: 10.1111/j.1432-1033.1972.tb02087.x. [DOI] [PubMed] [Google Scholar]
- Smuckler E. A., Hadjiolov A. A. Inhibition of hepatic deoxyribonucleic acid-dependent ribonucleic acid polymerases by the exotoxin of Bacillus thuringiensis in comparison with the effects of -amanitin and cordycepin. Biochem J. 1972 Aug;129(1):153–166. doi: 10.1042/bj1290153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAKAHASHI T., SWINT R. B., HURLBERT R. B. SYNTHESIS OF RNA IN ISOLATED NUCLEI OF THE NOVIKOFF ASCITES TUMOR. Exp Cell Res. 1963;24:SUPPL9–SUPPL9:344. doi: 10.1016/0014-4827(63)90274-x. [DOI] [PubMed] [Google Scholar]
- TSANEV R., STAINOV D. METOD PRIAMO I SPEKTROFOTOMETRII AGAROVYKH I KRAKHMAL'NYKH 'ELEKTROFOREGRAMM V UL'TRAFIOLETOVO I OBLASTI. Biokhimiia. 1964 Nov-Dec;29:1126–1131. [PubMed] [Google Scholar]
- Venkov P. V., Hadjiolov A. A. Characterization of rat liver nuclear ribonucleic acid fractions obtained by thermal phenol fractionation. Biochim Biophys Acta. 1967 Jun 20;142(1):276–279. doi: 10.1016/0005-2787(67)90538-2. [DOI] [PubMed] [Google Scholar]
- Weinberg R. A., Penman S. Processing of 45 s nucleolar RNA. J Mol Biol. 1970 Jan 28;47(2):169–178. doi: 10.1016/0022-2836(70)90337-2. [DOI] [PubMed] [Google Scholar]
- Wellauer P. K., Dawid I. B. Secondary structure maps of RNA: processing of HeLa ribosomal RNA. Proc Natl Acad Sci U S A. 1973 Oct;70(10):2827–2831. doi: 10.1073/pnas.70.10.2827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widnell C. C., Tata J. R. A procedure for the isolation of enzymically active rat-liver nuclei. Biochem J. 1964 Aug;92(2):313–317. doi: 10.1042/bj0920313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widnell C. C., Tata J. R. Studies on the stimulation by ammonium sulphate of the DNA-dependent RNA polymerase of isolated rat-liver nuclei. Biochim Biophys Acta. 1966 Sep;123(3):478–492. doi: 10.1016/0005-2787(66)90216-4. [DOI] [PubMed] [Google Scholar]
- Willems M., Wagner E., Laing R., Penman S. Base composition of ribosomal RNA precursors in the HeLa cell nucleolus: further evidence of non-conservative processing. J Mol Biol. 1968 Mar 14;32(2):211–220. doi: 10.1016/0022-2836(68)90005-3. [DOI] [PubMed] [Google Scholar]
- Wintersberger U., Smith P., Letnansky K. Yeast chromatin. Preparation from isolated nuclei, histone composition and transcription capacity. Eur J Biochem. 1973 Feb 15;33(1):123–130. doi: 10.1111/j.1432-1033.1973.tb02663.x. [DOI] [PubMed] [Google Scholar]
- Younger L. R., Gelboin H. V. The electrophoretic distribution of RNA synthesized in vitro by isolated rat liver nuclei. Biochim Biophys Acta. 1970 Mar 19;204(1):168–174. doi: 10.1016/0005-2787(70)90499-5. [DOI] [PubMed] [Google Scholar]
- Zylber E. A., Penman S. Products of RNA polymerases in HeLa cell nuclei. Proc Natl Acad Sci U S A. 1971 Nov;68(11):2861–2865. doi: 10.1073/pnas.68.11.2861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- al-Arif A., Sporn M. B. 2'-O-methylation of adenosine, guanosine, uridine, and cytidine in RNA of isolated rat liver nuclei. Proc Natl Acad Sci U S A. 1972 Jul;69(7):1716–1719. doi: 10.1073/pnas.69.7.1716. [DOI] [PMC free article] [PubMed] [Google Scholar]


