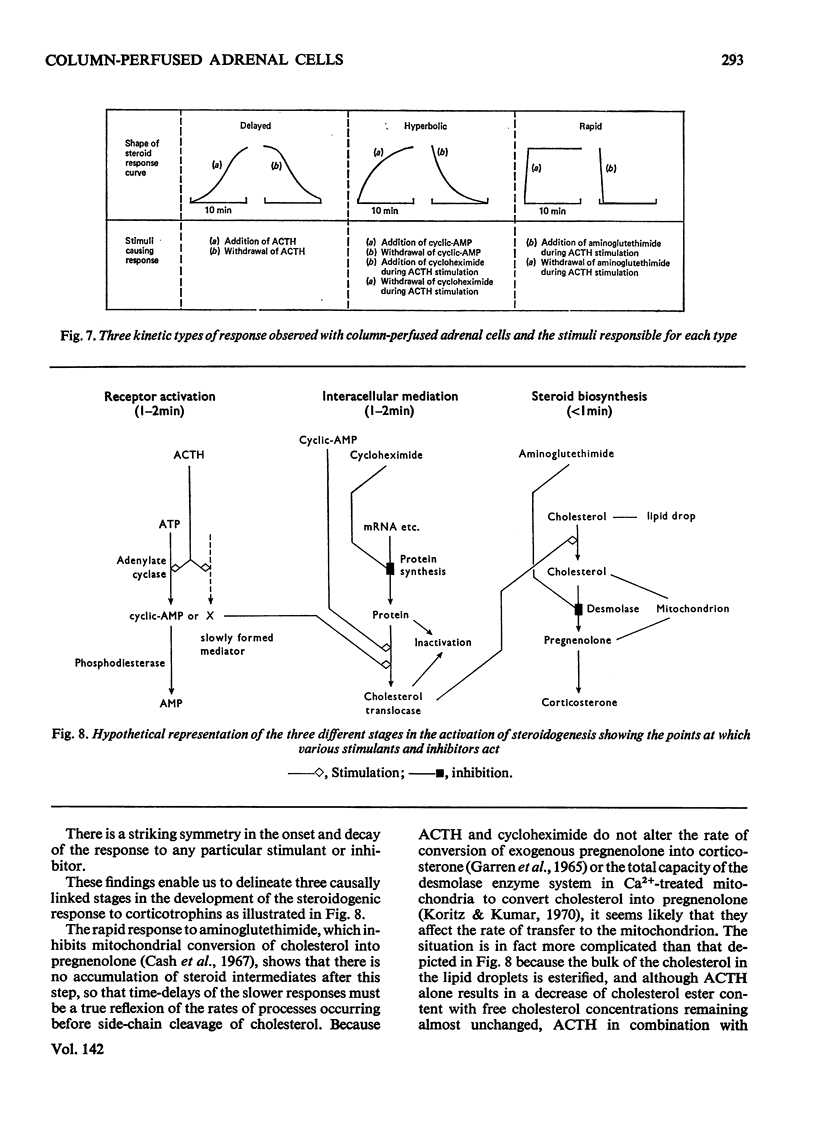
Abstract
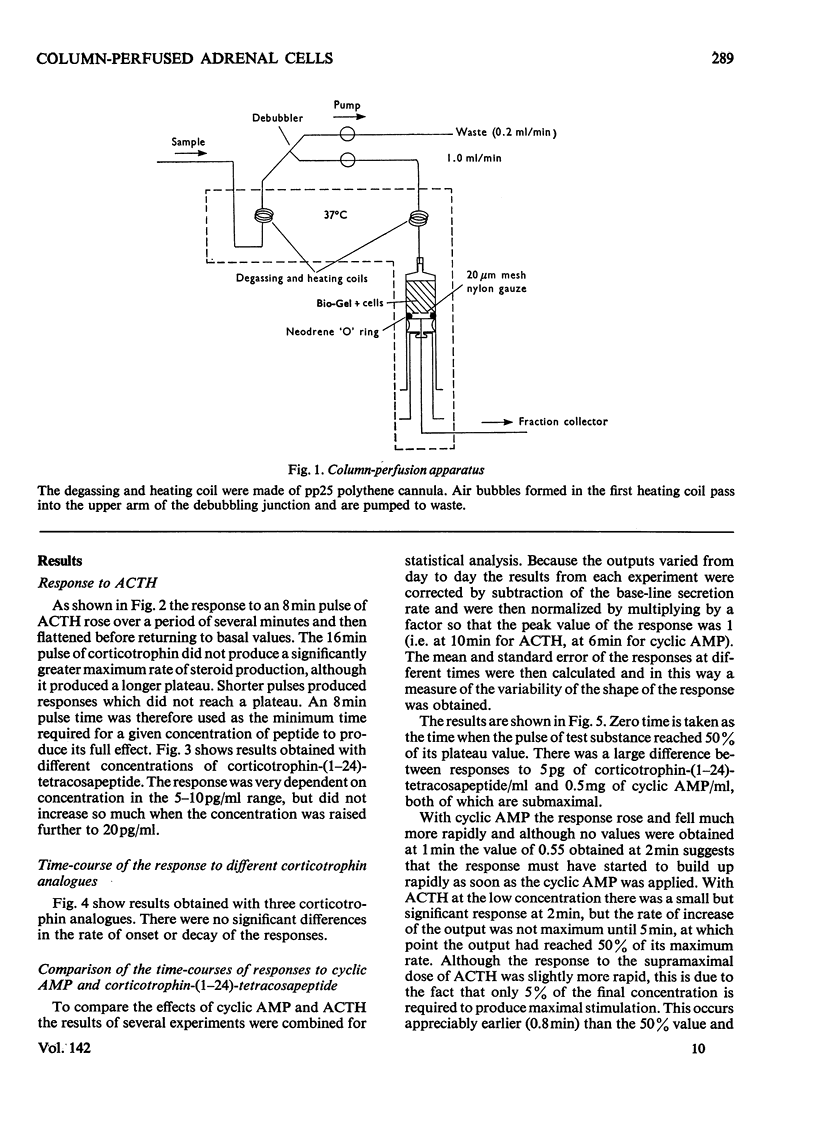
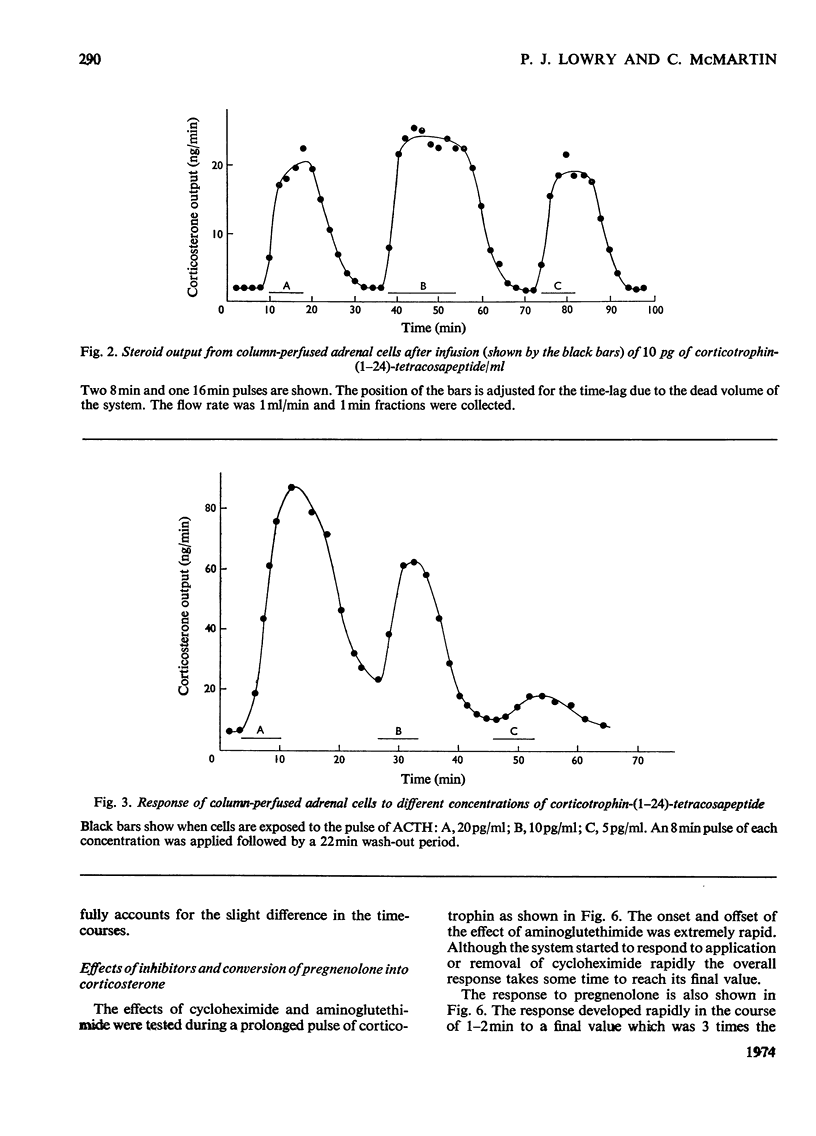
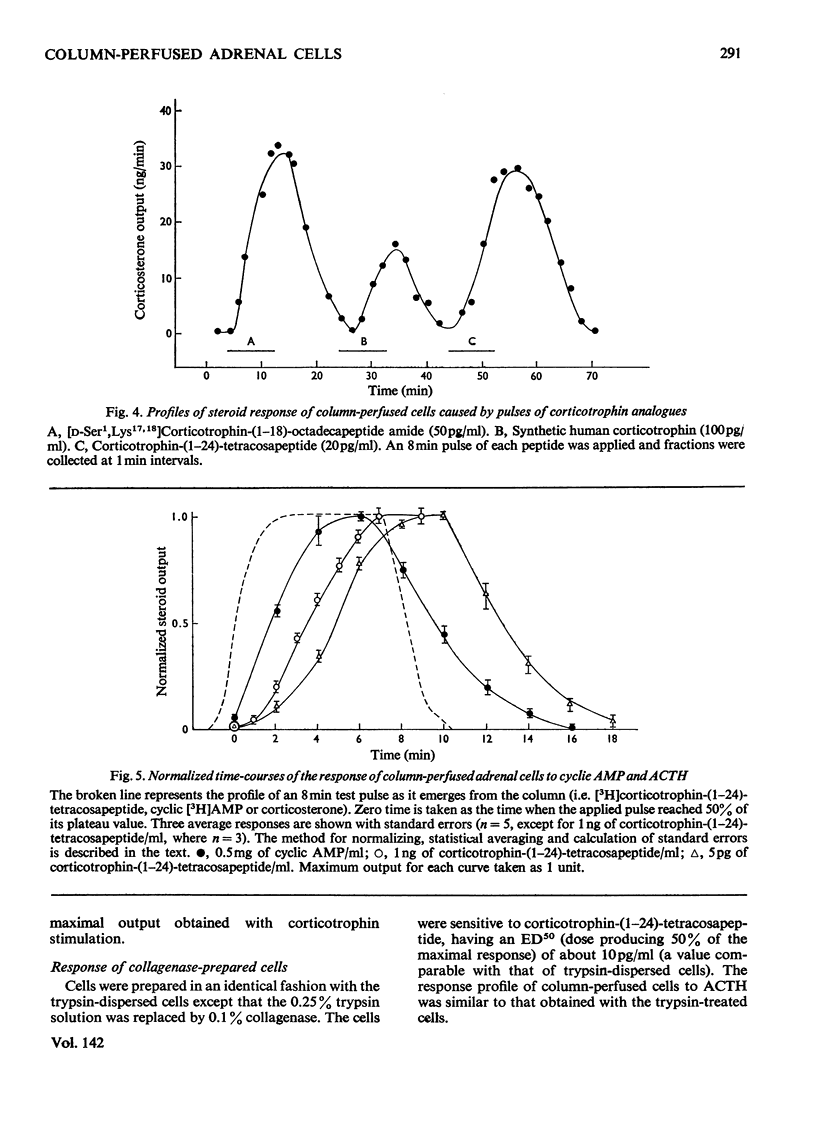
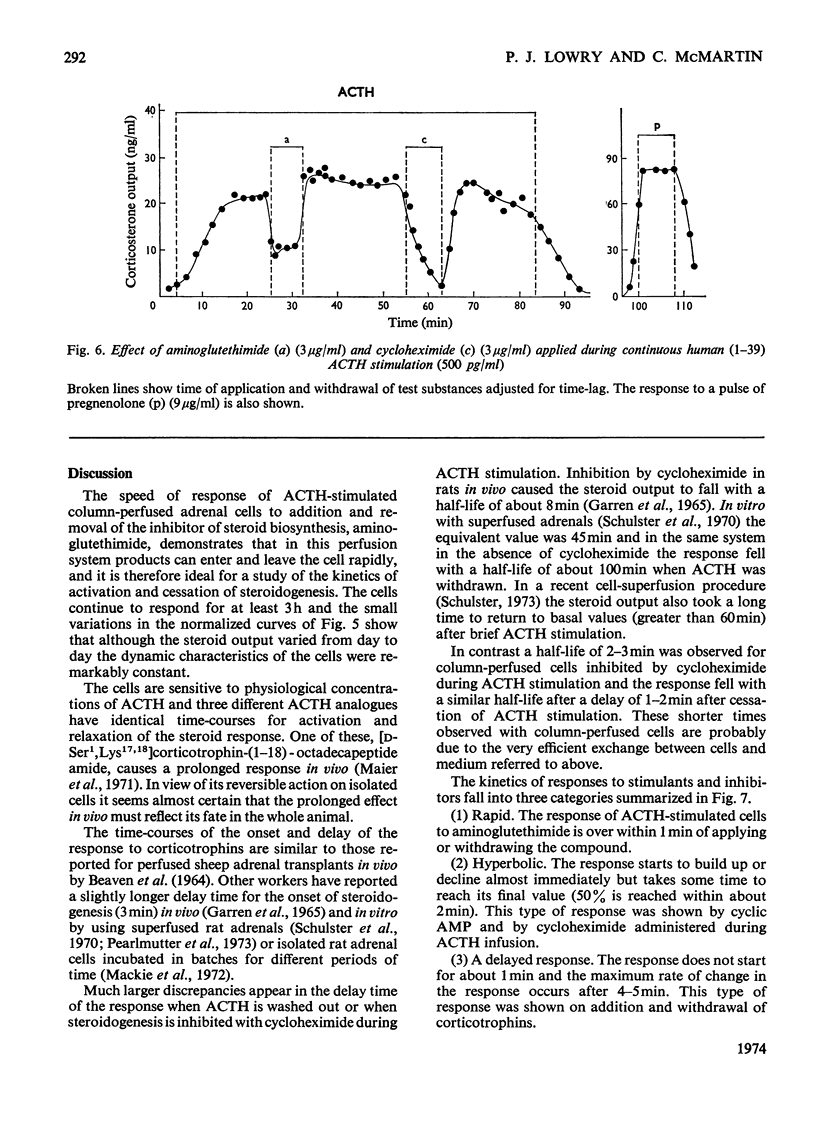
Isolated adrenal cells were perfused in a small column by using Bio-Gel polyacrylamide beads as an inert supporting matrix, and the time-course of the response to various stimuli was observed by measuring fluorogenic 11-hydroxycorticosteroids in the effluent. A small but significant response was observed 1 min after stimulation with physiological concentrations of ACTH (adrenocorticotrophin), but the response did not start to build up rapidly for 3–4min and eventually reached a plateau after 9–10min. A similar pattern of events was observed for the decay of the steroid output on removal of ACTH. ACTH analogues, including one with a long duration of action in vivo, were found to produce responses with similar kinetics. However, cyclic AMP caused a more rapid increase in steroidogenesis and its effects were more short-lived after withdrawal. If, as present evidence suggests, cyclic AMP is produced rapidly after ACTH stimulation the delayed build-up of the steroidogenic response to ACTH would indicate that cyclic AMP may not be the intracellular mediator. When inhibitors were applied during ACTH stimulation, aminoglutethimide, which blocks mitochondrial conversion of cholesterol into pregnenolone (3β-hydroxypregn-5-en-20-one), caused a rapid fall in steroid output (1 min), whereas cycloheximide took longer to achieve its full effect. Nevertheless, the response had fallen by 50% in 2 min, indicating a much shorter half-life than that previously reported for the labile protein implicated in steroidogenesis. In addition the rapid response to cyclic AMP makes it unlikely that steroid production is induced as a result of initiation of protein synthesis. This suggests that the labile protein plays an obligatory but permissive role in the development of the response. Column perfusion has proved to be a simple technique which can readily yield accurate data on responses of cells to stimulants and inhibitors.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BEAVEN D. W., ESPINER E. A., HART D. S. THE SUPPRESSION OF CORTISOL SECRETION BY STEROIDS, AND RESPONSE TO CORTICOTROPHIN, IN SHEEP WITH ADRENAL TRANSPLANTS. J Physiol. 1964 Jun;171:216–230. doi: 10.1113/jphysiol.1964.sp007373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett H. P., Bullock G., Lowry P. J., McMartin C., Peters J. Fate of corticotrophins in an isolated adrenal-cell bioassay and decrease of peptide breakdown by cell purification. Biochem J. 1974 Feb;138(2):185–194. doi: 10.1042/bj1380185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cash R., Brough A. J., Cohen M. N., Satoh P. S. Aminoglutethimide (Elipten-Ciba) as an inhibitor of adrenal steroidogenesis: mechanism of action and therapeutic trial. J Clin Endocrinol Metab. 1967 Sep;27(9):1239–1248. doi: 10.1210/jcem-27-9-1239. [DOI] [PubMed] [Google Scholar]
- FERGUSON J. J., Jr PROTEIN SYNTHESIS AND ADRENOCORTICOTROPIN RESPONSIVENESS. J Biol Chem. 1963 Aug;238:2754–2759. [PubMed] [Google Scholar]
- Garren L. D., Gill G. N., Masui H., Walton G. M. On the mechanism of action of ACTH. Recent Prog Horm Res. 1971;27:433–478. doi: 10.1016/b978-0-12-571127-2.50035-3. [DOI] [PubMed] [Google Scholar]
- Garren L. D., Ney R. L., Davis W. W. Studies on the role of protein synthesis in the regulation of corticosterone production by adrenocorticotropic hormone in vivo. Proc Natl Acad Sci U S A. 1965 Jun;53(6):1443–1450. doi: 10.1073/pnas.53.6.1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grahame-Smith D. G., Butcher R. W., Ney R. L., Sutherland E. W. Adenosine 3',5'-monophosphate as the intracellular mediator of the action of adrenocorticotropic hormone on the adrenal cortex. J Biol Chem. 1967 Dec 10;242(23):5535–5541. [PubMed] [Google Scholar]
- HAYNES R. C., Jr, KORITZ S. B., PERON F. G. Influence of adenosine 3',5'-monophosphate on corticoid production by rat adrenal glands. J Biol Chem. 1959 Jun;234(6):1421–1423. [PubMed] [Google Scholar]
- IMURA H., MATSUKURA S., MATSUYAMA H., SETSUDA T., MIYAKE T. ADRENAL STEROIDOGENIC EFFECT OF ADENOSINE 3',5'-MONOPHOSPHATE AND ITS DERIVATIVES IN VIVO. Endocrinology. 1965 May;76:933–937. doi: 10.1210/endo-76-5-933. [DOI] [PubMed] [Google Scholar]
- Koritz S. B., Kumar A. M. On the mechanism of action of the adrenocorticotrophic hormone. The stimulation of the activity of enzymes involved in pregnenolone synthesis. J Biol Chem. 1970 Jan 10;245(1):152–159. [PubMed] [Google Scholar]
- Lowry P. J., McMartin C., Peters J. Properties of a simplified bioassay for adrenocorticotrophic activity using the steroidogenic response of isolated adrenal cells. J Endocrinol. 1973 Oct;59(1):43–55. doi: 10.1677/joe.0.0590043. [DOI] [PubMed] [Google Scholar]
- Mackie C., Richardson M. C., Schulster D. Kinetics and dose-response characteristics of adenosine 3',5'-monophosphate production by isolated rat adrenal cells stimulated with adrenocorticotrophic hormone. FEBS Lett. 1972 Jul 1;23(3):345–348. doi: 10.1016/0014-5793(72)80312-0. [DOI] [PubMed] [Google Scholar]
- Pearlmutter A. F., Rapino E., Saffran M. Comparison of steroidogenic effects of cAMP and dbcAMP in the rat adrenal gland. Endocrinology. 1973 Mar;92(3):679–686. doi: 10.1210/endo-92-3-679. [DOI] [PubMed] [Google Scholar]
- Riniker B., Sieber P., Rittel W., Zuber H. Revised amino-acid sequences for porcine and human adrenocorticotrophic hormone. Nat New Biol. 1972 Jan 26;235(56):114–115. doi: 10.1038/newbio235114b0. [DOI] [PubMed] [Google Scholar]
- SILBER R. H., BUSCH R. D., OSLAPAS R. Practical procedure for estimation of corticosterone or hydrocortisone. Clin Chem. 1958 Aug;4(4):278–285. [PubMed] [Google Scholar]
- Sayers G., Swallow R. L., Giordano N. D. An improved technique for the preparation of isolated rat adrenal cells: a sensitive, accurate and specfic method for the assay of ACTH. Endocrinology. 1971 Apr;88(4):1063–1068. doi: 10.1210/endo-88-4-1063. [DOI] [PubMed] [Google Scholar]
- Schulster D. Regulation of steroidogenesis by ACTH in a superfusion system for isolated adrenal cells. Endocrinology. 1973 Sep;93(3):700–704. doi: 10.1210/endo-93-3-700. [DOI] [PubMed] [Google Scholar]
- Schulster D., Tait S. A., Tait J. F., Mrotek J. Production of steroids by in vitro superfusion of endocrine tissue. 3. Corticosterone output from rat adrenals stimulated by adrenocorticotropin or cyclic 3',5'-adenosine monophosphate and the inhibitory effect of cycloheximide. Endocrinology. 1970 Mar;86(3):487–502. doi: 10.1210/endo-86-3-487. [DOI] [PubMed] [Google Scholar]


