Abstract
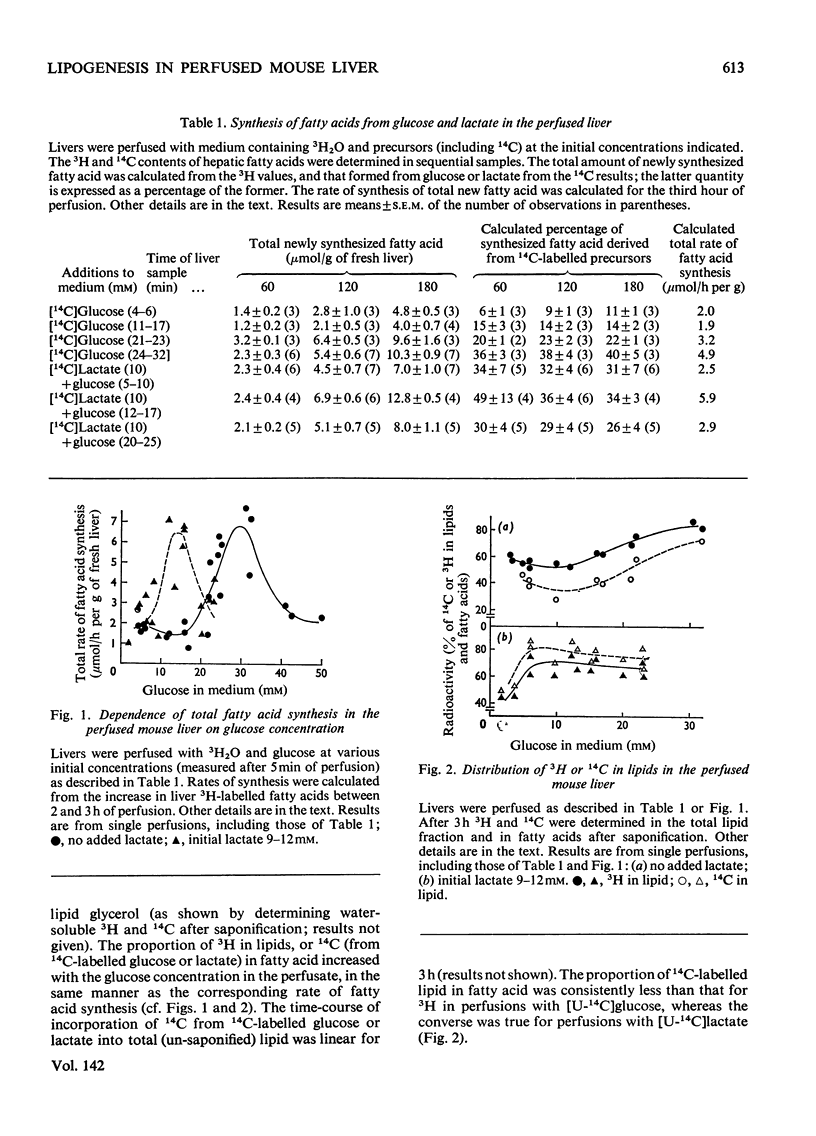
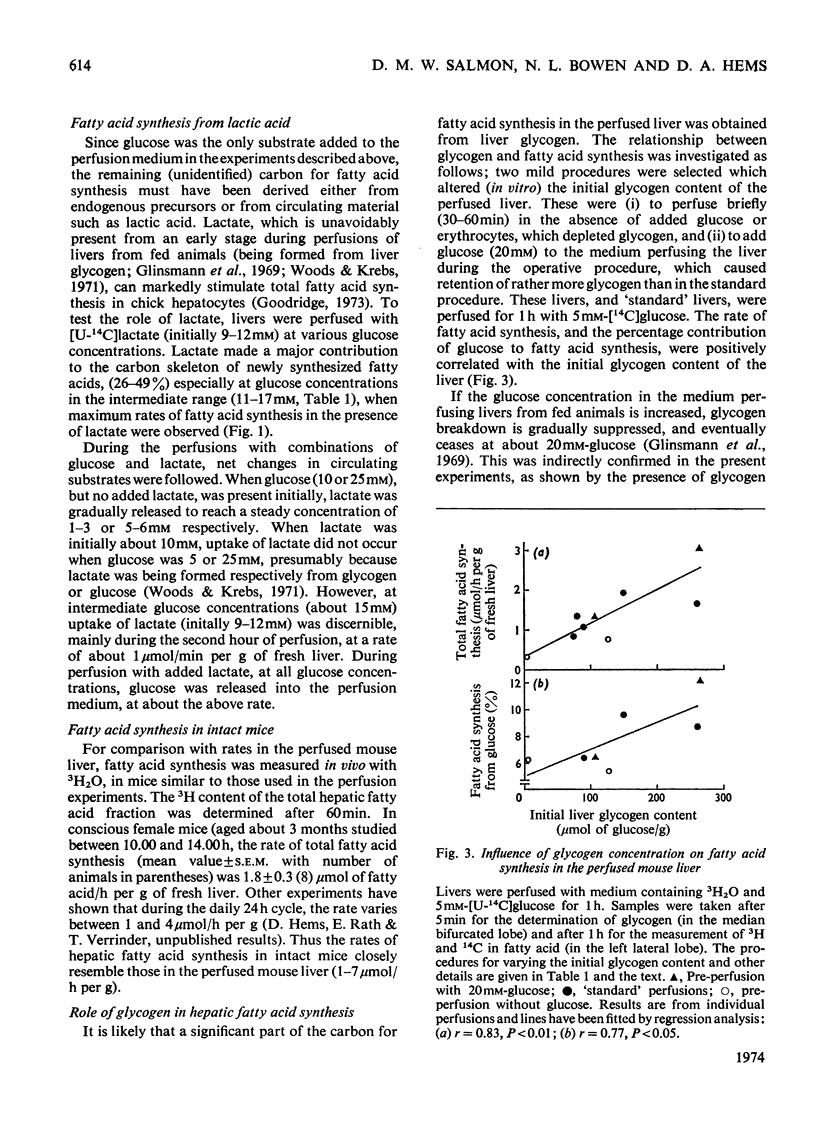
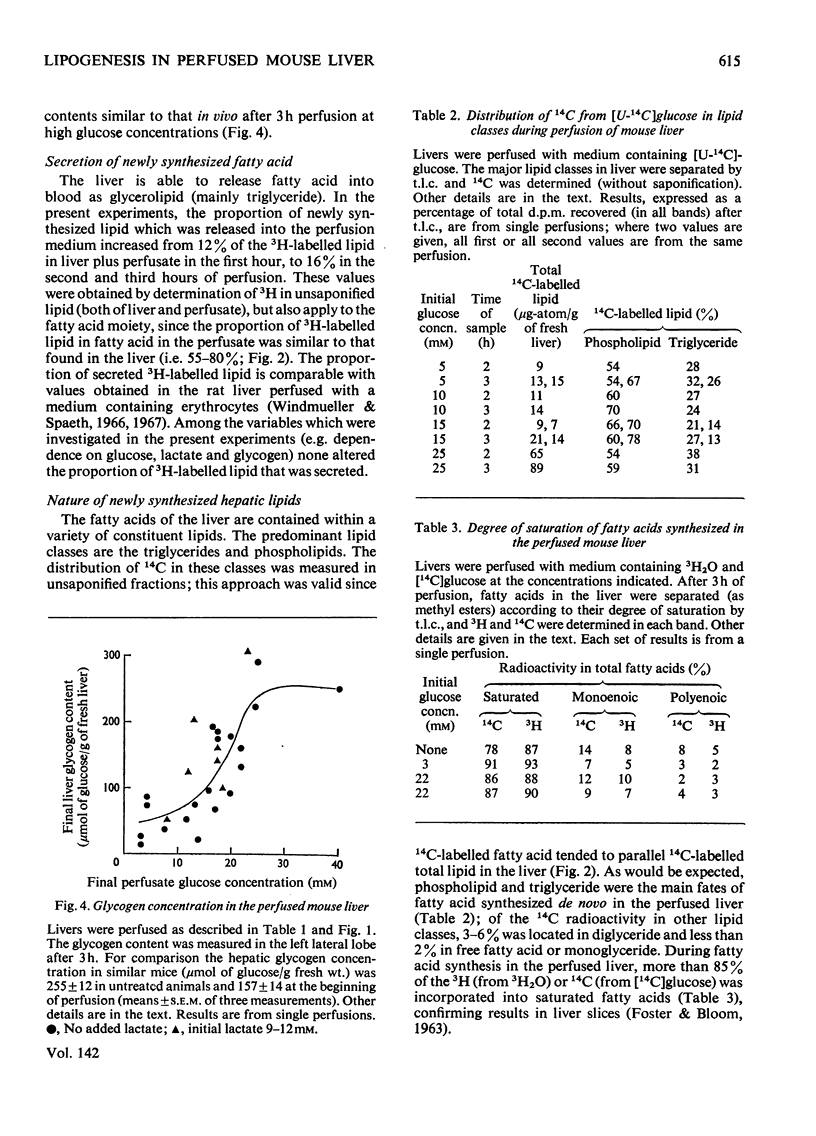
1. Fatty acid synthesis de novo was measured in the perfused liver of fed mice. 2. The total rate, measured by the incorporation into fatty acid of 3H from 3H2O (1–7μmol of fatty acid/h per g of fresh liver), resembled the rate found in the liver of intact mice. 3. Perfusions with l-[U-14C]lactic acid and [U-14C]glucose showed that circulating glucose at concentrations less than about 17mm was not a major carbon source for newly synthesized fatty acid, whereas lactate (10mm) markedly stimulated fatty acid synthesis, and contributed extensive carbon to lipogenesis. 4. The identification of 50% of the carbon converted into newly synthesized fatty acid lends further credibility to the use of 3H2O to measure hepatic fatty acid synthesis. 5. The total rate of fatty acid synthesis, and the contribution of glucose carbon to lipogenesis, were directly proportional to the initial hepatic glycogen concentration. 6. The proportion of total newly synthesized lipid that was released into the perfusion medium was 12–16%. 7. The major products of lipogenesis were saturated fatty acids in triglyceride and phospholipid. 8. The rate of cholesterol synthesis, also measured with 3H2O, expressed as acetyl residues consumed, was about one-fourth of the basal rate of fatty acid synthesis. 9. These results are discussed in terms of the carbon sources of hepatic newly synthesized fatty acids, and the effect of glucose, glycogen and lactate in stimulating lipogenesis, independently of their role as precursors.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BAILLIE L. A. Determination of liquid scientillation counting efficiency by pulse height shift. Int J Appl Radiat Isot. 1960 May;8:1–7. doi: 10.1016/0020-708x(60)90153-8. [DOI] [PubMed] [Google Scholar]
- Brunengraber H., Boutry M., Lowenstein J. M. Fatty acid and 3- -hydroxysterol synthesis in the perfused rat liver. Including measurements on the production of lactate, pyruvate, -hydroxy-butyrate, and acetoacetate by the fed liver. J Biol Chem. 1973 Apr 25;248(8):2656–2669. [PubMed] [Google Scholar]
- Brunengraber H., Sabine J. R., Boutry M., Lowenstein J. M. 3- -Hydroxysterol synthesis by the liver. Arch Biochem Biophys. 1972 Jun;150(2):392–396. doi: 10.1016/0003-9861(72)90054-9. [DOI] [PubMed] [Google Scholar]
- Clark D. G., Rognstad R., Katz J. Lipogenesis in rat hepatocytes. J Biol Chem. 1974 Apr 10;249(7):2028–2036. [PubMed] [Google Scholar]
- DIPIETRO D. L., SHARMA C., WEINHOUSE S. Studies on glucose phosphorylation in rat liver. Biochemistry. 1962 May 25;1:455–462. doi: 10.1021/bi00909a014. [DOI] [PubMed] [Google Scholar]
- DUNN E., ROBSON P. QUANTITATIVE GRAVIMETRIC ANALYSIS OF FATTY ESTER MIXTURES BY THIN-LAYER CHROMATOGRAPHY. J Chromatogr. 1965 Mar;17:501–505. doi: 10.1016/s0021-9673(00)99901-1. [DOI] [PubMed] [Google Scholar]
- Elliott J., Dade E., Salmon D. M., Hems D. A. Hepatic metabolism in normal and genetically obese mice. Biochim Biophys Acta. 1974 Apr 22;343(2):307–323. doi: 10.1016/0304-4165(74)90095-6. [DOI] [PubMed] [Google Scholar]
- Elliott J., Hems D. A., Beloff-Chain A. Carbohydrate metabolism of the isolated perfused liver of normal and genetically obese--hyperglycaemic (ob-ob) mice. Biochem J. 1971 Dec;125(3):773–780. doi: 10.1042/bj1250773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Exton J. H., Corbin J. G., Harper S. C. Control of gluconeogenesis in liver. V. Effects of fasting, diabetes, and glucagon on lactate and endogenous metabolism in the perfused rat liver. J Biol Chem. 1972 Aug 25;247(16):4996–5003. [PubMed] [Google Scholar]
- Exton J. H. Gluconeogenesis. Metabolism. 1972 Oct;21(10):945–990. doi: 10.1016/0026-0495(72)90028-5. [DOI] [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- FOSTER D. W., BLOOM B. The synthesis of fatty acids by rat liver slices in tritiated water. J Biol Chem. 1963 Mar;238:888–892. [PubMed] [Google Scholar]
- Glinsmann W. H., Hern E. P., Lynch A. Intrinsic regulation of glucose output by rat liver. Am J Physiol. 1969 Apr;216(4):698–703. doi: 10.1152/ajplegacy.1969.216.4.698. [DOI] [PubMed] [Google Scholar]
- Goodridge A. G. Regulation of fatty acid synthesis in isolated hepatocytes prepared from the livers of neonatal chicks. J Biol Chem. 1973 Mar 25;248(6):1924–1931. [PubMed] [Google Scholar]
- HAUGAARD E. S., STADIE W. C. Relation between glycogen content and synthesis of fatty acids by rat liver. J Biol Chem. 1952 Dec;199(2):741–744. [PubMed] [Google Scholar]
- HENDLER R. W. PROCEDURE FOR SIMULTANEOUS ASSAY OF TWO BETA-EMITTING ISOTOPES WITH THE LIQUID SCINTILLATION COUNTING TECHNIQUE. Anal Biochem. 1964 Jan;7:110–120. doi: 10.1016/0003-2697(64)90125-3. [DOI] [PubMed] [Google Scholar]
- Hems D. A., Whitton P. D. Stimulation by vasopressin of glycogen breakdown and gluconeogenesis in the perfused rat liver. Biochem J. 1973 Nov;136(3):705–709. doi: 10.1042/bj1360705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hems D. A., Whitton P. D., Taylor E. A. Glycogen synthesis in the perfused liver of the starved rat. Biochem J. 1972 Sep;129(3):529–538. doi: 10.1042/bj1290529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hers H. G., De Wulf H., Stalmans W., van den Berghe G. The control of glycogen synthesis in the liver. Adv Enzyme Regul. 1970;8:171–190. doi: 10.1016/0065-2571(70)90016-6. [DOI] [PubMed] [Google Scholar]
- Jungas R. L. Fatty acid synthesis in adipose tissue incubated in tritiated water. Biochemistry. 1968 Oct;7(10):3708–3717. doi: 10.1021/bi00850a050. [DOI] [PubMed] [Google Scholar]
- Lowenstein J. M. Effect of (-)-hydroxycitrate on fatty acid synthesis by rat liver in vivo. J Biol Chem. 1971 Feb 10;246(3):629–632. [PubMed] [Google Scholar]
- Mayes P. A., Topping D. L. Regulation of hepatic lipogenesis by plasma free fatty acids: simultaneous studies on lipoprotein secretion, cholesterol synthesis, ketogenesis and gluconeogenesis. Biochem J. 1974 Apr;140(1):111–114. doi: 10.1042/bj1400111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGarry J. D., Foster D. W. The regulation of ketogenesis from octanoic acid. The role of the tricarboxylic acid cycle and fatty acid synthesis. J Biol Chem. 1971 Feb 25;246(4):1149–1159. [PubMed] [Google Scholar]
- McGarry J. D., Meier J. M., Foster D. W. The effects of starvation and refeeding on carbohydrate and lipid metabolism in vivo and in the perfused rat liver. The relationship between fatty acid oxidation and esterification in the regulation of ketogenesis. J Biol Chem. 1973 Jan 10;248(1):270–278. [PubMed] [Google Scholar]
- Salmon D. M., Hems D. A. Plasma lipoproteins and the synthesis and turnover of plasma triglyceride in normal and genetically obese mice. Biochem J. 1973 Nov;136(3):551–563. doi: 10.1042/bj1360551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topping D. L., Mayes P. A. The immediate effects of insulin and fructose on the metabolism of the perfused liver. Changes in lipoprotein secretion, fatty acid oxidation and esterification, lipogenesis and carbohydrate metabolism. Biochem J. 1972 Jan;126(2):295–311. doi: 10.1042/bj1260295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker D. G., Rao S. The role of glucokinase in the phosphorylation of glucose by rat liver. Biochem J. 1964 Feb;90(2):360–368. doi: 10.1042/bj0900360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windmueller H. G., Spaeth A. E. De novo synthesis of fatty acid in perfused rat liver as a determinant of plasma lipoprotein production. Arch Biochem Biophys. 1967 Nov;122(2):362–369. doi: 10.1016/0003-9861(67)90206-8. [DOI] [PubMed] [Google Scholar]
- Windmueller H. G., Spaeth A. E. Perfusion in situ with tritium oxide to measure hepatic lipogenesis and lipid secretion. Normal and orotic acid-fed rats. J Biol Chem. 1966 Jun 25;241(12):2891–2899. [PubMed] [Google Scholar]
- Woods H. F., Krebs H. A. Lactate production in the perfused rat liver. Biochem J. 1971 Nov;125(1):129–139. doi: 10.1042/bj1250129. [DOI] [PMC free article] [PubMed] [Google Scholar]


