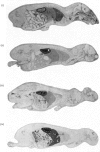
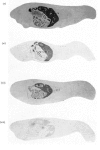
Abstract
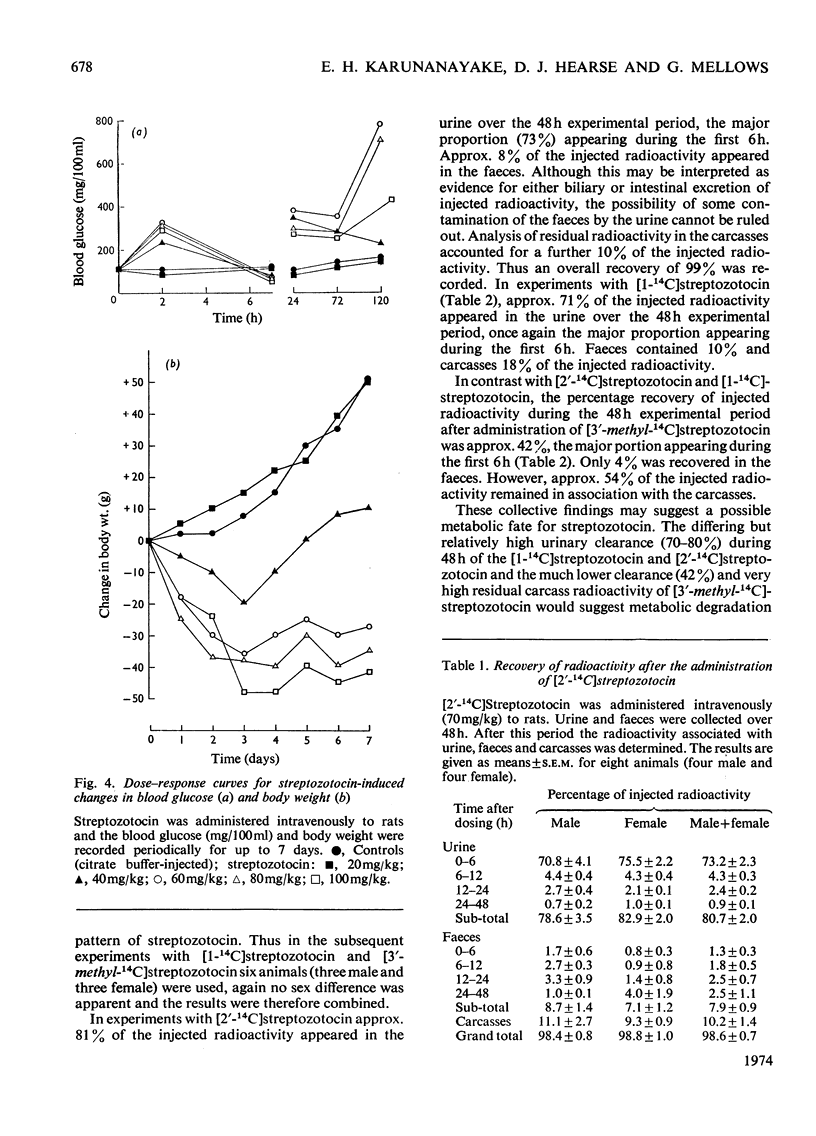
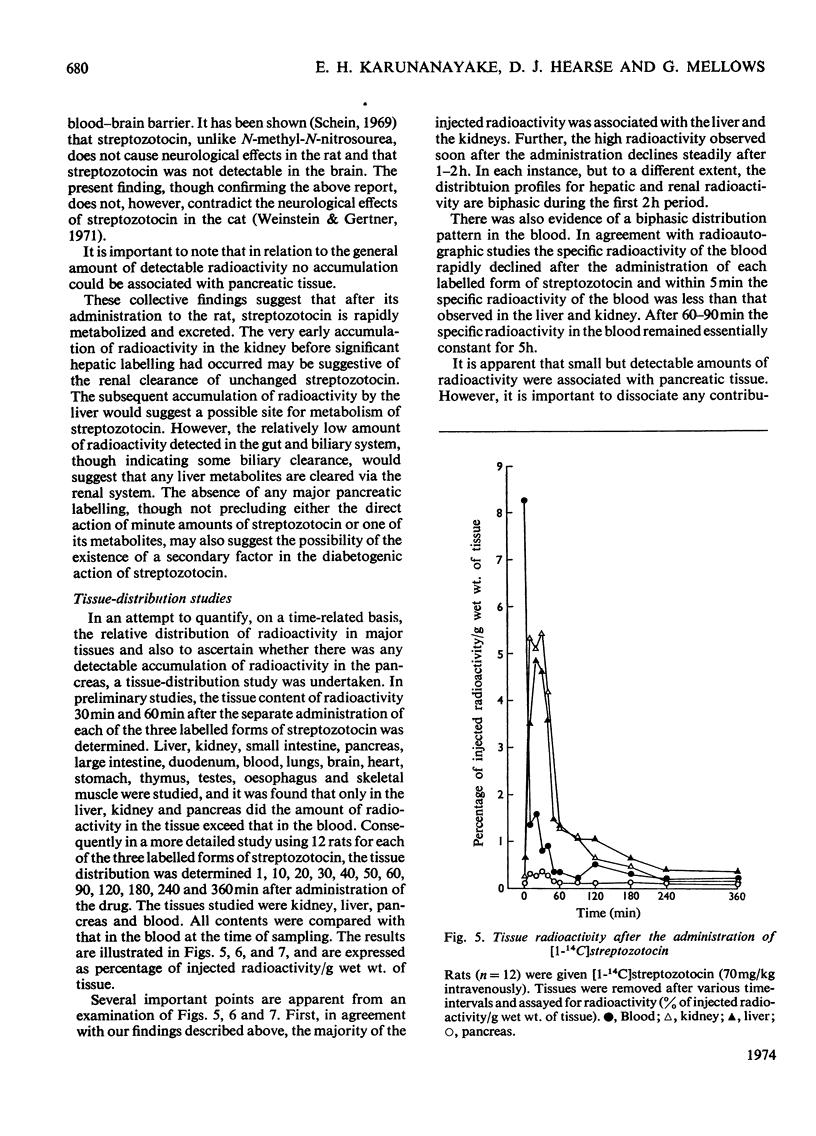
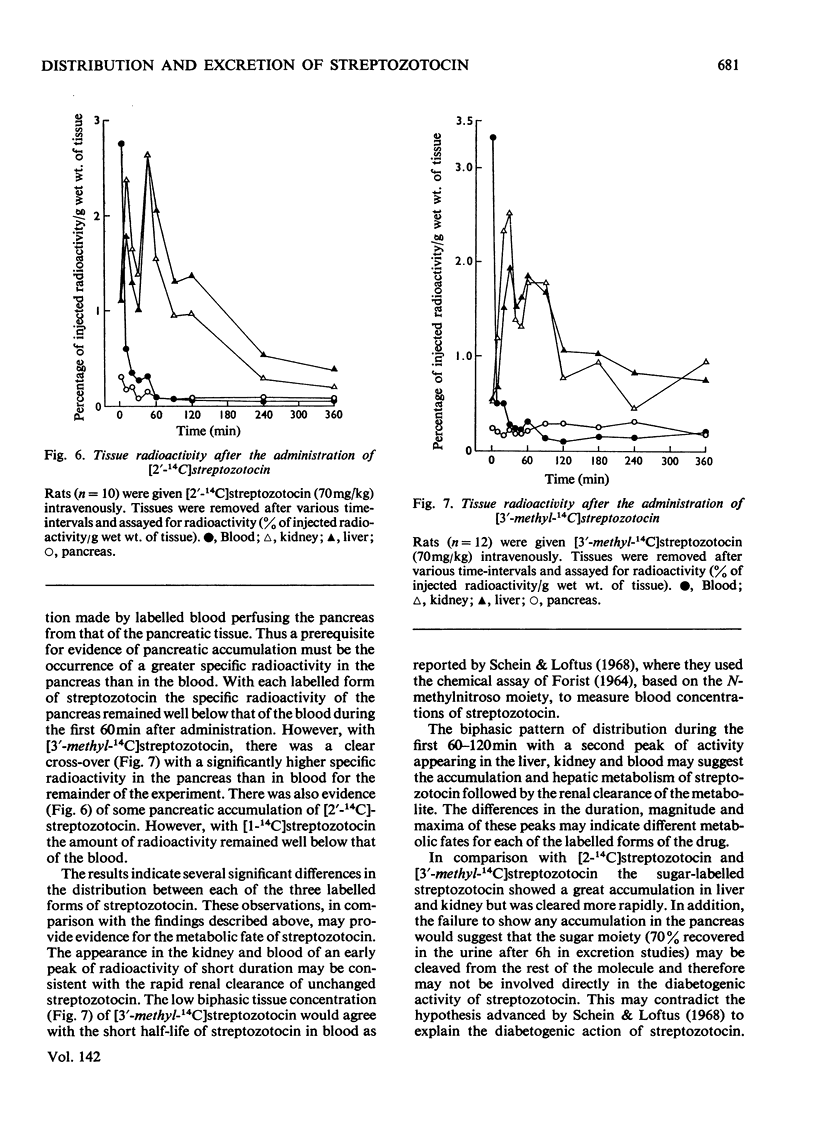
[14C]Streptozotocin was synthesized specifically labelled at three positions in the molecule. The biological activity of synthetic streptozotocin was characterised by studies in vivo of its diabetogenic activity and its dose–response curves. After this characterization the excretion pattern of all three labelled forms of streptozotocin was studied. With [1-14C]streptozotocin and [2′-14C]streptozotocin the injected radioactivity was excreted (approx. 70% and 80% respectively) mainly in the urine, the greater part of the excretion occurring in the first 6h period; small amounts (approx. 9% and 8% respectively) were found in the faeces. In contrast, with [3′-methyl-14C]streptozotocin a much smaller proportion (approx. 42%) of the injected radioactivity was excreted in the urine, the major proportion appearing in the first 6h, whereas approx. 53% of the injected radioactivity was retained in the carcasses. In whole-body radioautographic studies very rapid renal clearance and hepatic accumulation of the injected radioactivity was observed with all three labelled forms of the drug. There was some evidence for biliary and intestinal excretion. Major differences were apparent in the tissue-distribution studies, with each of the three labelled forms, particularly with [3′-methyl-14C]streptozotocin. There was no accumulation of [1-14C]streptozotocin in the pancreas for the 6h period after administration. However, with [3′-methyl-14C]streptozotocin (and also [2′-14C]streptozotocin) there was evidence of some pancreatic accumulation after 2h. The results indicate that streptozotocin is subjected to considerable metabolic transformation and to rapid renal clearance. The implication of these suggestions is evaluated with particular reference to the diabetogenic action of streptozotocin.
Full text
PDF


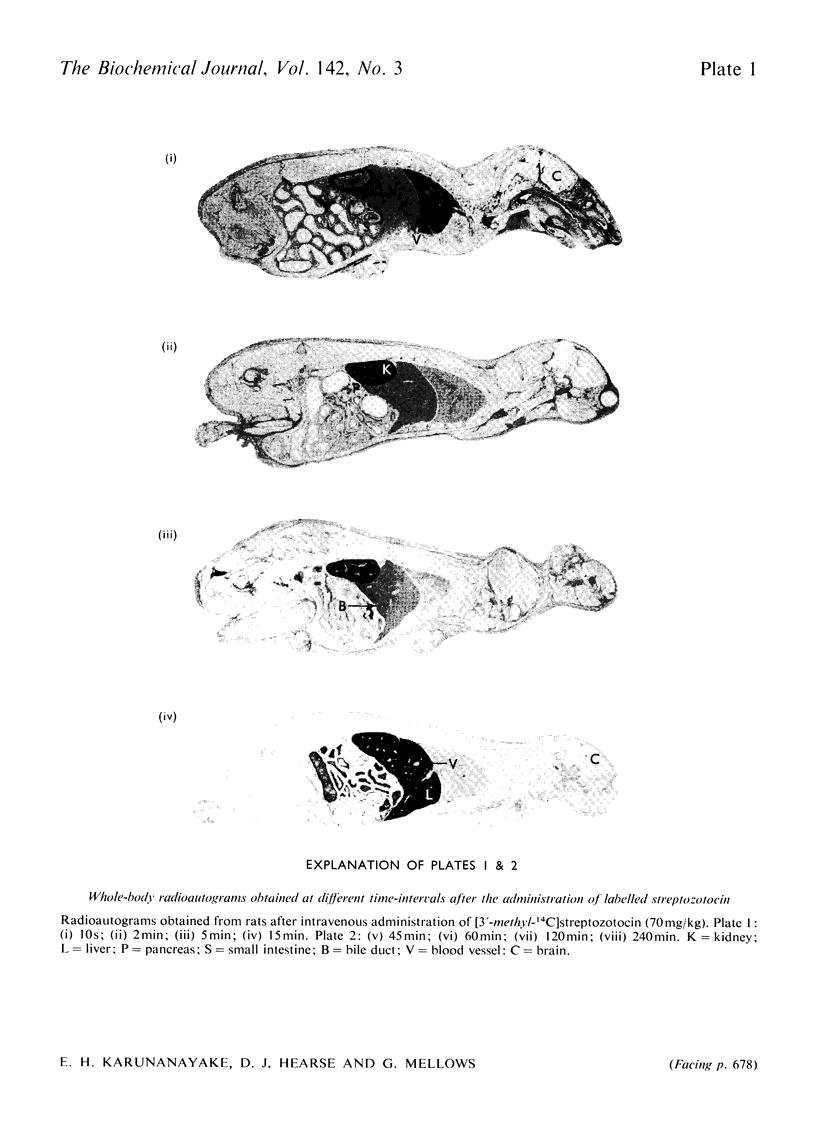
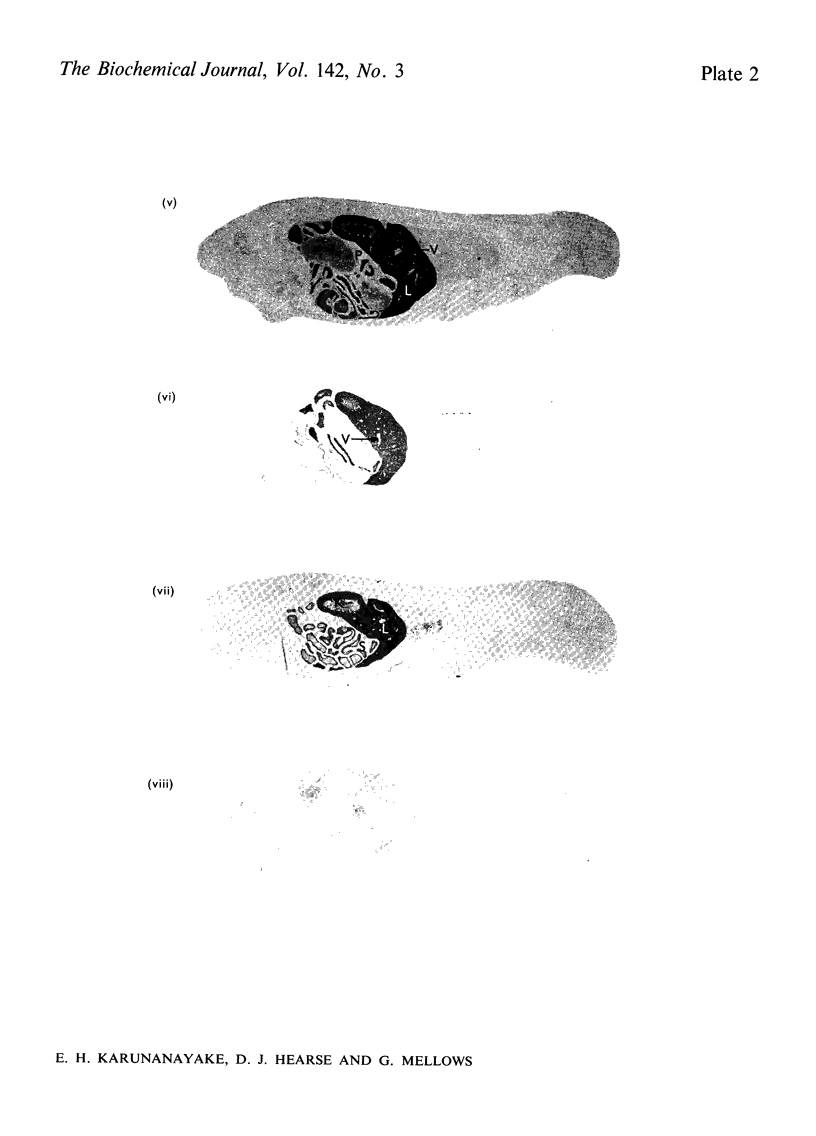



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arison R. N., Ciaccio E. I., Glitzer M. S., Cassaro J. A., Pruss M. P. Light and electron microscopy of lesions in rats rendered diabetic with streptozotocin. Diabetes. 1967 Jan;16(1):51–56. doi: 10.2337/diab.16.1.51. [DOI] [PubMed] [Google Scholar]
- Arison R. N., Feudale E. L. Induction of renal tumour by streptozotocin in rats. Nature. 1967 Jun 17;214(5094):1254–1255. doi: 10.1038/2141254a0. [DOI] [PubMed] [Google Scholar]
- Bannister B. The synthesis and biological activities of some analogs of streptozotocin. J Antibiot (Tokyo) 1972 Jul;25(7):377–386. doi: 10.7164/antibiotics.25.377. [DOI] [PubMed] [Google Scholar]
- Bhuyan B. K., Fraser T. J., Buskirk H. H., Neil G. L. Antileukemic activity of streptozotocin (NSC-85998) and its analogs. Cancer Chemother Rep. 1972 Dec;56(6):709–720. [PubMed] [Google Scholar]
- Bridges J. W., Davies D. S., Williams R. T. The fate of ethyltin and diethyltin derivatives in the rat. Biochem J. 1967 Dec;105(3):1261–1267. doi: 10.1042/bj1051261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspary W. F., Rhein A. M., Creutzfeldt W. Increase of intestinal brush border hydrolases in mucosa of streptozotocin-diabetic rats. Diabetologia. 1972 Dec;8(6):412–414. doi: 10.1007/BF01212169. [DOI] [PubMed] [Google Scholar]
- Dixit P. K., Tam B. B., Hernandez R. E. Comparison of the effect of diabetogenic agents on the microdissected pancreatic islet tissue of the rat. 1. Proc Soc Exp Biol Med. 1972 Sep;140(4):1418–1423. doi: 10.3181/00379727-140-36687. [DOI] [PubMed] [Google Scholar]
- Dulin W. E., Wyse B. M. Reversal of streptozotocin diabetes with nicotinamide. Proc Soc Exp Biol Med. 1969 Mar;130(3):992–994. doi: 10.3181/00379727-130-33707. [DOI] [PubMed] [Google Scholar]
- Dulin W. E., Wyse B. M. Studies on the ability of compounds to block the diabetogenic activity of streptozotocin. Diabetes. 1969 Jul;18(7):459–466. doi: 10.2337/diab.18.7.459. [DOI] [PubMed] [Google Scholar]
- Evans J. S., Gerritsen G. C., Mann K. M., Owen S. P. Antitumor and hyperglycemic activity of streptozotocin (NSC-37917) and its cofactor, U-15,774. Cancer Chemother Rep. 1965 Oct;48:1–6. [PubMed] [Google Scholar]
- HALES C. N., RANDLE P. J. Immunoassay of insulin with insulin-antibody precipitate. Biochem J. 1963 Jul;88:137–146. doi: 10.1042/bj0880137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUGGETT A. S., NIXON D. A. Use of glucose oxidase, peroxidase, and O-dianisidine in determination of blood and urinary glucose. Lancet. 1957 Aug 24;273(6991):368–370. doi: 10.1016/s0140-6736(57)92595-3. [DOI] [PubMed] [Google Scholar]
- Herr R. R., Jahnke J. K., Argoudelis A. D. The structure of streptozotocin. J Am Chem Soc. 1967 Aug 30;89(18):4808–4809. doi: 10.1021/ja00994a053. [DOI] [PubMed] [Google Scholar]
- Hessler E. J., Jahnke H. K. Improved synthesis of streptozotocin. J Org Chem. 1970 Jan;35(1):245–246. doi: 10.1021/jo00826a053. [DOI] [PubMed] [Google Scholar]
- Ho C. K., Hashim S. A. Pyridine nucleotide depletion in pancreatic islets associated with streptozotocin-induced diabetes. Diabetes. 1972 Jul;21(7):789–793. doi: 10.2337/diab.21.7.789. [DOI] [PubMed] [Google Scholar]
- Hoftiezer V., Carpenter A. M. Comparison of streptozotocin and alloxan-induced diabetes in the rat, including volumetric quantitation of the pancreatic islets. Diabetologia. 1973 Jun;9(3):178–184. doi: 10.1007/BF01219780. [DOI] [PubMed] [Google Scholar]
- Ichiyama A., Nakamura S., Nishizuka Y. Studies on the biosynthesis of nicotinamide adenine dinucleotide (NAD) in mammals and its regulatory mechanism. I. Arzneimittelforschung. 1967 Nov;17(11):1346–1355. [PubMed] [Google Scholar]
- Ichiyama A., Nakamura S., Nishizuka Y. Studies on the biosynthesis of nicotinamide adenine dinucleotide (NAD) in mammals and its regulatory mechanism. II. Arzneimittelforschung. 1967 Dec;17(12):1525–1530. [PubMed] [Google Scholar]
- Junod A., Lambert A. E., Stauffacher W., Renold A. E. Diabetogenic action of streptozotocin: relationship of dose to metabolic response. J Clin Invest. 1969 Nov;48(11):2129–2139. doi: 10.1172/JCI106180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarus S. S., Shapiro S. H. Influence of nicotinamide and pyridine nucleotides on streptozotocin and alloxan-induced pancreatic B cell cytotoxicity. Diabetes. 1973 Jul;22(7):499–506. doi: 10.2337/diab.22.7.499. [DOI] [PubMed] [Google Scholar]
- Lazarus S. S., Shapiro S. H. Streptozotocin-induced diabetes and islet cell alterations in rabbits. Diabetes. 1972 Mar;21(3):129–137. doi: 10.2337/diab.21.3.129. [DOI] [PubMed] [Google Scholar]
- Mansford K. R., Opie L. Comparison of metabolic abnormalities in diabetes mellitus induced by streptozotocin or by alloxan. Lancet. 1968 Mar 30;1(7544):670–671. doi: 10.1016/s0140-6736(68)92103-x. [DOI] [PubMed] [Google Scholar]
- Moertel C. G., Reitemeier R. J., Schutt A. J., Hahn R. G. Phase II study of strepozotocin (NSC-85998) in the treatment of advanced gastrointestinal cancer. Cancer Chemother Rep. 1971 Jun;55(3):303–307. [PubMed] [Google Scholar]
- NARROD S. A., BONAVITA V., EHRENFELD E. R., KAPLAN N. O. Effect of azaserine on the biosynthesis of diphosphopyridine nucleotide in mouse. J Biol Chem. 1961 Mar;236:931–935. [PubMed] [Google Scholar]
- PREISS J., HANDLER P. Biosynthesis of diphosphopyridine nucleotide. I. Identification of intermediates. J Biol Chem. 1958 Aug;233(2):488–492. [PubMed] [Google Scholar]
- Pearson J. W., Gibson W. T., Chirigos M. A. Use of a murine sarcoma virus in an in vivo assay for antiviral and antitumor agents. Cancer Res. 1970 Jul;30(7):2024–2028. [PubMed] [Google Scholar]
- Pegg A. E., Williams-Ashman H. G. Biosynthesis of putrescine in the prostate gland of the rat. Biochem J. 1968 Jul;108(4):533–539. doi: 10.1042/bj1080533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersson B., Hellerström C., Gunnarsson R. Structure and metabolism of the pancreatic islets in streptozotocin treated guinea pigs. Horm Metab Res. 1970 Nov;2(6):313–317. doi: 10.1055/s-0028-1095064. [DOI] [PubMed] [Google Scholar]
- Pitkin R. M., Reynolds W. A. Diabetogenic effects of streptozotocin in rhesus monkeys. Diabetes. 1970 Feb;19(2):85–90. doi: 10.2337/diab.19.2.85. [DOI] [PubMed] [Google Scholar]
- RAKIETEN N., RAKIETEN M. L., NADKARNI M. V. Studies on the diabetogenic action of streptozotocin (NSC-37917). Cancer Chemother Rep. 1963 May;29:91–98. [PubMed] [Google Scholar]
- Rerup C. C. Drugs producing diabetes through damage of the insulin secreting cells. Pharmacol Rev. 1970 Dec;22(4):485–518. [PubMed] [Google Scholar]
- Rerup C., Tarding F. Streptozotocin- and alloxan-diabetes in mice. Eur J Pharmacol. 1969 Jul;7(1):89–96. doi: 10.1016/0014-2999(69)90169-1. [DOI] [PubMed] [Google Scholar]
- Reusser F. Mode of action of streptozotocin. J Bacteriol. 1971 Feb;105(2):580–588. doi: 10.1128/jb.105.2.580-588.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schein P. S. 1-methyl-1-nitrosourea depression of brain nicotinamide adenine dinucleotide in the production of neurologic toxicity. Proc Soc Exp Biol Med. 1969 Jun;131(2):517–520. doi: 10.3181/00379727-131-33914. [DOI] [PubMed] [Google Scholar]
- Schein P. S., Bates R. W. Plasma glucose levels in normal and adrenalectomized mice treated with streptozotocin and nicotinamide. Diabetes. 1968 Dec;17(12):760–765. doi: 10.2337/diab.17.12.760. [DOI] [PubMed] [Google Scholar]
- Schein P. S., Cooney D. A., McMenamin M. G., Anderson T. Streptozotocin diabetes--further studies on the mechanism of depression of nicotinamide adenine dinucleotide concentrations in mouse pancreatic islets and liver. Biochem Pharmacol. 1973 Oct 15;22(20):2625–2631. doi: 10.1016/0006-2952(73)90071-3. [DOI] [PubMed] [Google Scholar]
- Schein P. S., Cooney D. A., Vernon M. L. The use of nicotinamide to modify the toxicity of streptozotocin diabetes without loss of antitumor activity. Cancer Res. 1967 Dec;27(12):2324–2332. [PubMed] [Google Scholar]
- Schein P. S., Loftus S. Streptozotocin: depression of mouse liver pyridine nucleotides. Cancer Res. 1968 Aug;28(8):1501–1506. [PubMed] [Google Scholar]
- Schreibman P. H., De Koliren L. G., Arky R. A. Metastatic insulinoma treated with streptozotocin. Ann Intern Med. 1971 Mar;74(3):399–403. doi: 10.7326/0003-4819-74-3-399. [DOI] [PubMed] [Google Scholar]
- Shioiri T., Ninomiya K., Yamada S. Diphenylphosphoryl azide. A new convenient reagent for a modified Curtus reaction and for the peptide synthesis. J Am Chem Soc. 1972 Aug 23;94(17):6203–6205. doi: 10.1021/ja00772a052. [DOI] [PubMed] [Google Scholar]
- Stauffacher W., Burr I., Gutzeit A., Beaven D., Veleminsky J., Renold A. E. Streptozotocin diabetes: time course of irreversible B-cell damage; further observations on prevention by nicotinamide. Proc Soc Exp Biol Med. 1970 Jan;133(1):194–200. doi: 10.3181/00379727-133-34439. [DOI] [PubMed] [Google Scholar]
- VAVRA J. J., DEBOER C., DIETZ A., HANKA L. J., SOKOLSKI W. T. Streptozotocin, a new antibacterial antibiotic. Antibiot Annu. 1959;7:230–235. [PubMed] [Google Scholar]
- Weinstein S., Gertner S. B. The neurological and metabolic effects of streptozotocin in the cat. Pharmacology. 1971;6(3):129–136. doi: 10.1159/000136235. [DOI] [PubMed] [Google Scholar]
- Wilander E., Boquist L. Streptozotocin-diabetes in the Chinese hamster. Blood glucose and structural changes during the first 24 hours. Horm Metab Res. 1972 Nov;4(6):426–433. doi: 10.1055/s-0028-1094000. [DOI] [PubMed] [Google Scholar]