Abstract
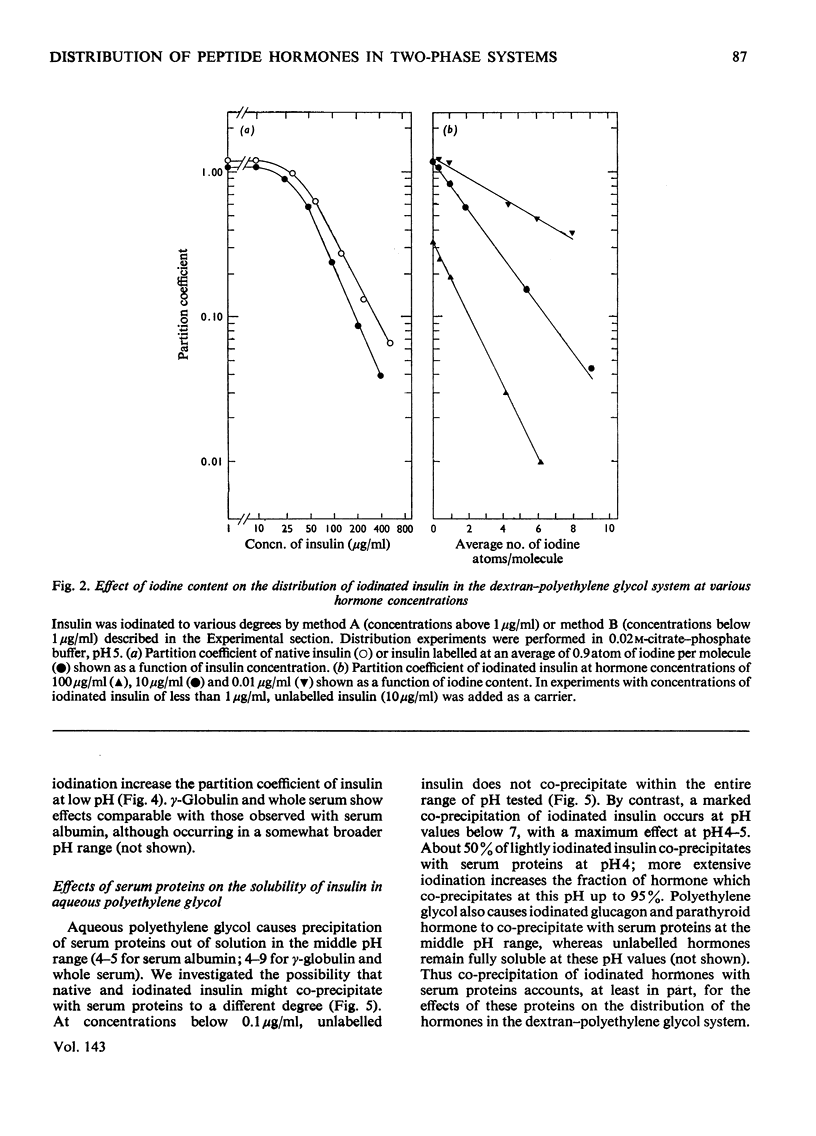
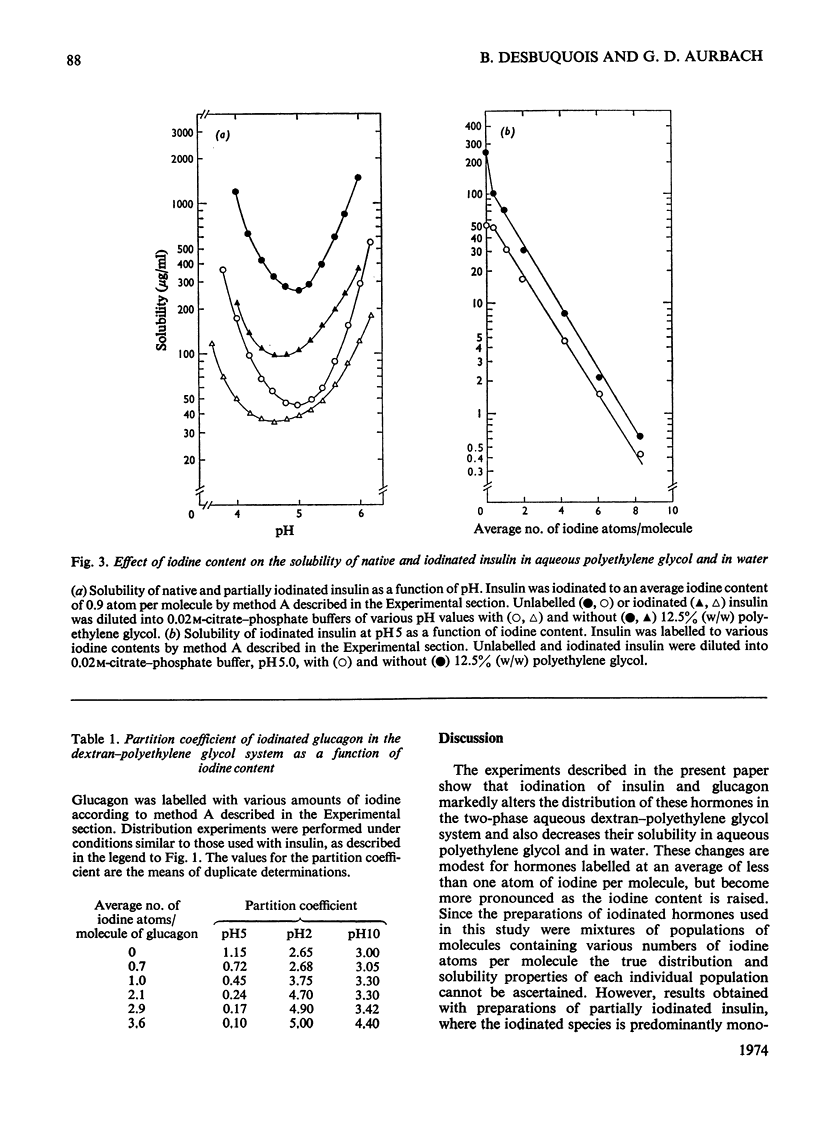
1. The effect of iodination on the distribution of peptide hormones into the aqueous two-phase dextran–polyethylene glycol system and on the solubility of these hormones in aqueous polyethylene glycol and in water was assessed. Hormones that were studied included insulin, glucagon and parathyroid hormone. 2. The partition coefficient of native insulin in the dextran–polyethylene glycol system showed a minimum (about 1) near the isoelectric point of the hormone (pH 5). Partial iodination of insulin (one atom per molecule) caused little change in the distribution of the hormone. More extensive iodination markedly decreased the partition coefficient in the region of the isoelectric point and displaced the pH value at which the partition coefficient was a minimum towards lower values. 3. The solubility of native insulin in aqueous polyethylene glycol and in water showed a pH-dependence similar to that observed for the distribution in the dextran–polyethylene glycol system. Iodination of insulin decreased the solubility of the hormone in polyethylene glycol and in water in parallel, and decreased the pH value at which solubility was a minimum. The changes in solubility correlated with the degree of iodination and accounted for the changes in distribution observed at high concentrations of insulin. 4. Comparable effects of iodination on distribution and solubility were also observed with glucagon. 5. At concentrations of insulin below its maximum solubility, serum proteins caused a decrease in the partition coefficient of iodinated hormone, but not of native hormone. These effects correlated with the degree of iodination and resulted from a co-precipitation of iodinated insulin with serum proteins.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albertsson P. A. Partition of cell particles and macromolecules in polymer two-phase systems. Adv Protein Chem. 1970;24:309–341. doi: 10.1016/s0065-3233(08)60244-2. [DOI] [PubMed] [Google Scholar]
- Berson S. A., Yalow R. S. Iodoinsulin used to determine specific activity of iodine-131. Science. 1966 Apr 8;152(3719):205–207. doi: 10.1126/science.152.3719.205. [DOI] [PubMed] [Google Scholar]
- Bromer W. W., Boucher M. E., Patterson J. M. Glucagon structure and function. II. Increased activity of iodoglucagon. Biochem Biophys Res Commun. 1973 Jul 2;53(1):134–139. doi: 10.1016/0006-291x(73)91411-3. [DOI] [PubMed] [Google Scholar]
- Desbuquois B., Aurbach G. D. Distribution of free and antibody-bound peptide hormones in two-phase aqueous polymer systems. Biochem J. 1972 Feb;126(3):717–726. doi: 10.1042/bj1260717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desbuquois B., Aurbach G. D. Use of polyethylene glycol to separate free and antibody-bound peptide hormones in radioimmunoassays. J Clin Endocrinol Metab. 1971 Nov;33(5):732–738. doi: 10.1210/jcem-33-5-732. [DOI] [PubMed] [Google Scholar]
- EDELHOCH H., LIPPOLDT R. E. The properties of thyroglobulin. IX. The molecular properties of iodinated thyroglobulin. J Biol Chem. 1962 Sep;237:2788–2794. [PubMed] [Google Scholar]
- Edelhoch H., Lippoldt R. E. Structural studies on polypeptide hormones. I. Fluorescence. J Biol Chem. 1969 Jul 25;244(14):3876–3883. [PubMed] [Google Scholar]
- HUNTER W. M., GREENWOOD F. C. Preparation of iodine-131 labelled human growth hormone of high specific activity. Nature. 1962 May 5;194:495–496. doi: 10.1038/194495a0. [DOI] [PubMed] [Google Scholar]
- Herbert V., Lau K. S., Gottlieb C. W., Bleicher S. J. Coated charcoal immunoassay of insulin. J Clin Endocrinol Metab. 1965 Oct;25(10):1375–1384. doi: 10.1210/jcem-25-10-1375. [DOI] [PubMed] [Google Scholar]
- Juckes I. R. Fractionation of proteins and viruses with polyethylene glycol. Biochim Biophys Acta. 1971 Mar 23;229(3):535–546. [PubMed] [Google Scholar]
- Perlman R. L., Edelhoch H. The formation of diiodotyrosine in iodinated human serum albumin. J Biol Chem. 1967 May 25;242(10):2416–2422. [PubMed] [Google Scholar]
- Rodbard D., Rayford P. L., Cooper J. A., Ross G. T. Statistical quality control of radioimmunoassays. J Clin Endocrinol Metab. 1968 Oct;28(10):1412–1418. doi: 10.1210/jcem-28-10-1412. [DOI] [PubMed] [Google Scholar]
- Simpson R. T., Vallee B. L. Iodocarboxypeptidase. Biochemistry. 1966 May;5(5):1760–1767. doi: 10.1021/bi00869a045. [DOI] [PubMed] [Google Scholar]


