Abstract
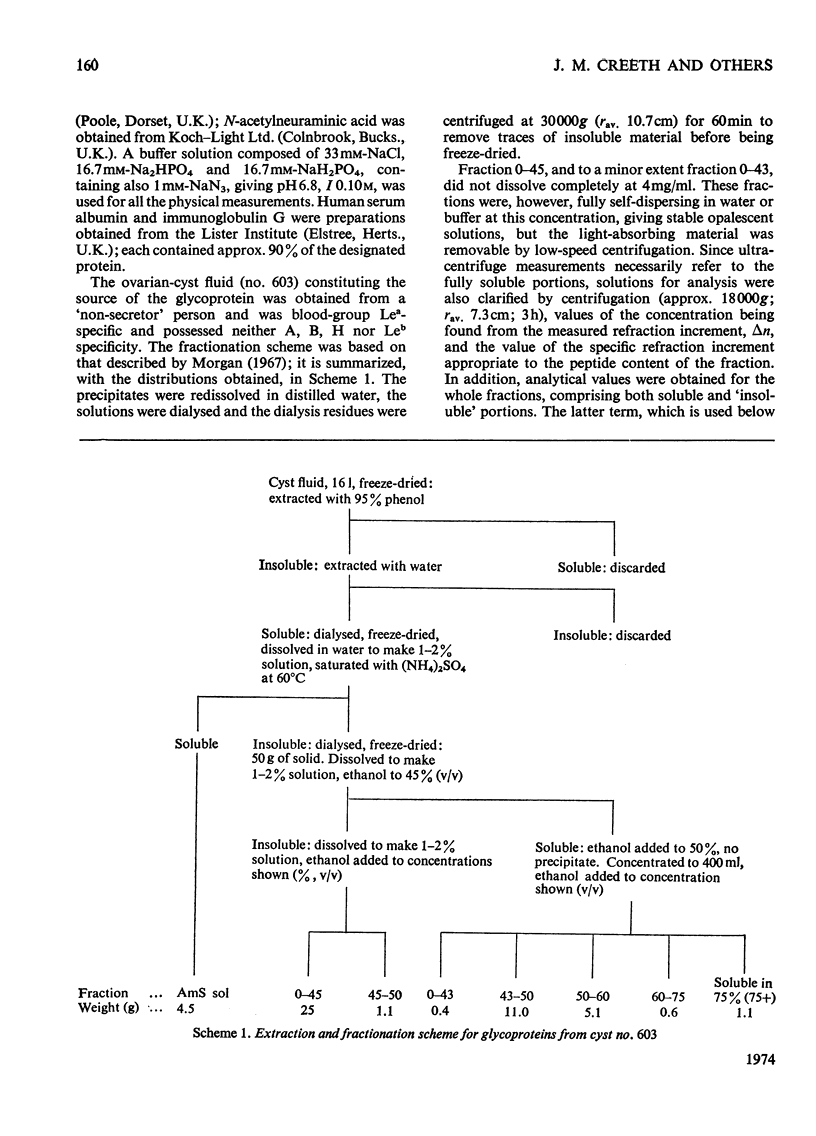
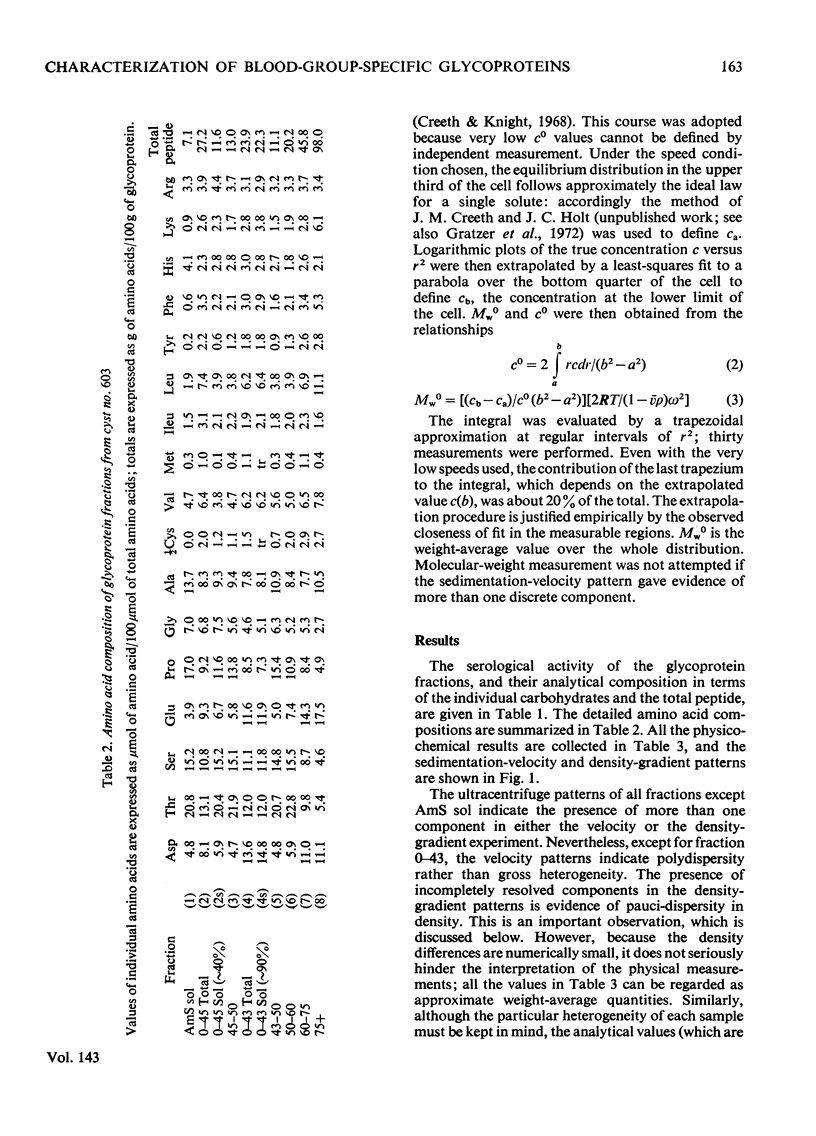
1. The glycoprotein components of a human ovarian-cyst fluid were isolated by a solvent [95% (w/w) phenol]-extraction procedure; the phenol-insoluble water-soluble glycoprotein was further fractionated by (NH4)2SO4 and by ethanol to yield eight fractions. 2. The fractions were analysed in terms of amino acids, fucose, galactose, N-acetylglucosamine, N-acetylgalactosamine and sialic acid. Variations occurred, particularly in the proportion of peptide; these were partly correlated with varying extent of serological activity. 3. The fractions were characterized physicochemically in terms of buoyant density and degree of spreading in a density gradient, sedimentation velocity and molecular weight; their partial specific volumes and specific refraction increments were also determined. 4. The fractions showed wide variations in their sedimentation-velocity and density-gradient patterns, and gave evidence of pauci-dispersity in density. The fraction regarded as the most typical blood-group-specific glycoprotein sedimented as a single rapidly spreading peak and was of high molecular weight. 5. Significant correlations were observed between the physical properties of the glycoprotein fractions and the amount of their peptide component. The buoyant densities and sedimentation coefficients varied in a manner that suggested the existence of two families of glycoproteins. 6. It is suggested that variability in the extent of glycosylation, or in the degree of cross-linking, might account for the two families of glycoproteins, and that the extent of cross-linkage might also be a factor determining the solubility of these glycoproteins in hot saturated (NH4)2SO4.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Creeth J. M., Denborough M. A. The use of equilibrium-density-gradient methods for the preparation and characterization of blood-group-specific glycoproteins. Biochem J. 1970 May;117(5):879–891. doi: 10.1042/bj1170879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creeth J. M., Knight C. G. The macromolecular properties of blood-group substances. Sedimentation-velocity and viscosity measurements. Biochem J. 1967 Dec;105(3):1135–1145. doi: 10.1042/bj1051135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denborough M. A., Creeth J. M. Density gradient studies of A, B, H and Lea blood group specific glycoproteins. Clin Chim Acta. 1970 Nov;30(2):447–451. doi: 10.1016/0009-8981(70)90138-5. [DOI] [PubMed] [Google Scholar]
- Dunstone J. R. Fractionation in a density gradient of some glycoproteins from human ovarian cyst fluids. Eur J Biochem. 1969 May 1;9(1):128–132. doi: 10.1111/j.1432-1033.1969.tb00585.x. [DOI] [PubMed] [Google Scholar]
- Dunstone J. R., Morgan W. T. Further observations on the glycoproteins in human ovarian cyst fluids. Biochim Biophys Acta. 1965 Nov 1;101(3):300–314. doi: 10.1016/0926-6534(65)90009-6. [DOI] [PubMed] [Google Scholar]
- Franek M. D., Dunstone J. R. Density-gradient centrifugation in the isolation of polysaccharide-protein complexes from aortic tissue. Biochim Biophys Acta. 1966 Sep 26;127(1):213–222. doi: 10.1016/0304-4165(66)90491-0. [DOI] [PubMed] [Google Scholar]
- Gratzer W. B., Creeth J. M., Beaven G. H. Presence ot trimers in glucagon solution. Eur J Biochem. 1972 Dec 18;31(3):505–509. doi: 10.1111/j.1432-1033.1972.tb02558.x. [DOI] [PubMed] [Google Scholar]
- HEIMBURGER N., HEIDE K., HAUPT H., SCHULTZE H. E. BAUSTEINANALYSEN VON HUMANSERUMPROTEINEN. Clin Chim Acta. 1964 Oct;10:293–307. doi: 10.1016/0009-8981(64)90059-2. [DOI] [PubMed] [Google Scholar]
- Hexner P. E., Radford L. E., Beams J. W. ACHIEVEMENT OF SEDIMENTATION EQUILIBRIUM. Proc Natl Acad Sci U S A. 1961 Nov;47(11):1848–1852. doi: 10.1073/pnas.47.11.1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristiansen T., Porath J. Studies on blood group substances. I. Purification of active material from hog gastric mucin by specific precipitation with Vicia cracca phytohemagglutinin. Biochim Biophys Acta. 1968 Jun 24;158(3):351–357. [PubMed] [Google Scholar]
- PUSZTAI A., MORGAN W. T. STUDIES IN IMMUNOCHEMISTRY. 22. THE AMINO ACID COMPOSITION OF THE HUMAN BLOOD-GROUP A, B, H AND LE-A SPECIFIC SUBSTANCES. Biochem J. 1963 Sep;88:546–555. doi: 10.1042/bj0880546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PUSZTAI A., MORGAN W. T. Studies in immunochemistry. 19. Further observations on the preparation and properties of human blood-group-specific mucopolysaccharides. Biochem J. 1961 Jul;80:107–121. doi: 10.1042/bj0800107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TANFORD C., MARLER E., JURY E., DAVIDSON E. A. DETERMINATION OF THE MOLECULAR WEIGHT DISTRIBUTION OF CHONDROITIN SULFATE B BY SEDIMENTATION EQUILIBRIUM. J Biol Chem. 1964 Dec;239:4034–4040. [PubMed] [Google Scholar]
- WAKE R. G., BALDWIN R. L. Physical studies on the replication of DNA in vitro. J Mol Biol. 1962 Aug;5:201–216. doi: 10.1016/s0022-2836(62)80084-9. [DOI] [PubMed] [Google Scholar]
- WARREN L. The thiobarbituric acid assay of sialic acids. J Biol Chem. 1959 Aug;234(8):1971–1975. [PubMed] [Google Scholar]




